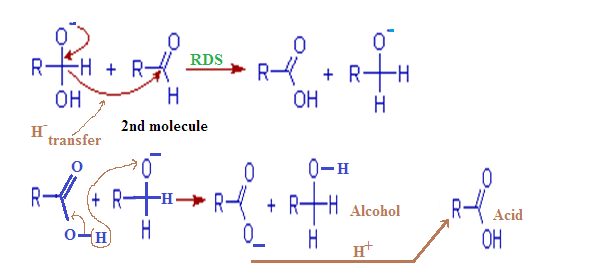
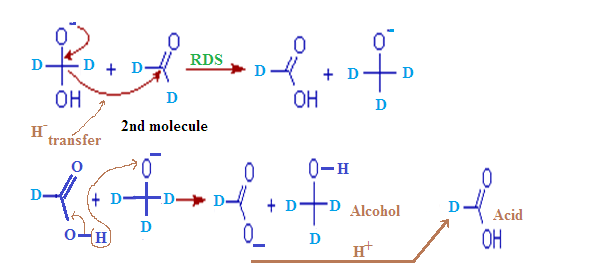
Step – (1):
The cannizzaro
reaction is initiated by the nucleophilic attack of a hydroxide ion to the
carbonyl carbon of an aldehyde molecule by giving a hydrate anion. This hydrate
anion can be deprotonated to give an anion in a strongly alkaline medium.
Step – (2):
In this second
step, the hydroxide behaves as a base. Now a hydride ion, H- is
transferred either from the mono anionic
species onto the carbonyl carbon of another
aldehyde molecule. The strong electron donating effect of O-
groups facilitates the hydride transfer and drives the reaction further. This is the
rate determining step of the reaction.
Thus
one molecule is oxidized to carboxylic acid and the other one is reduced to an
alcohol.