Dancing
resonance is a special stability mechanism which increases stability of carbocations
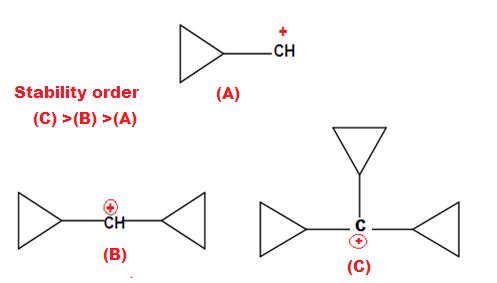
attached directly to the three membered rings. For example Cyclopropylmethyl Carbocation.
In
cyclopropane all the carbon is sp3 hybridized and the bond angle for the same
should be 109
degree 28′ but the actual bond angle is 60 due to which angle strain develops. So
in order to minimize the strain p orbital bents due to which it acquires
partial sigma and partial pi bond character which behave like pi bond. And resonance
take place between sigma and vacant p-orbitals hence called P-orbitals
overlapping or sigma resonance.
We know by
Drago’s rule bond angle is directly proportional to the s-character while inversely
proportional to p-character.
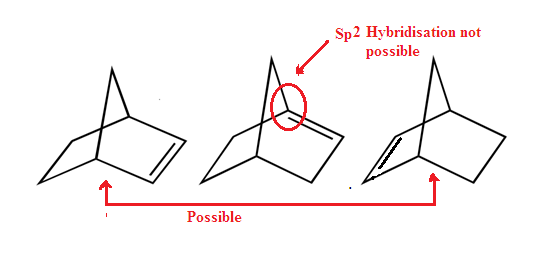
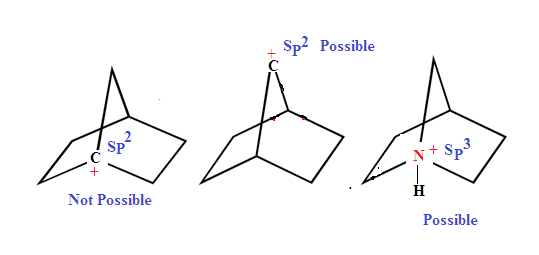
Dancing
resonance is a hypothetical phenomenon which is reduces strain of the ring,
hence the carbocation is more stable. CH2+
has a vacant p orbital and a very effective overlapping takes place between
p-orbital and electron density of cyclopropane, due to this its stability is
very high. There is a conjugation between the sigma bond and positive charge.
The
exceptional stability of cyclopropane methyl cation can be explained by the
concept of dancing resonance concept. The stability of additional cyclopropyl
group , is result of more conjugation between the bent orbital of cyclopropyl
ring and cationic carbon.
The
most stable carbocation known till date in organic chemistry is explain by
Dancing resonance.