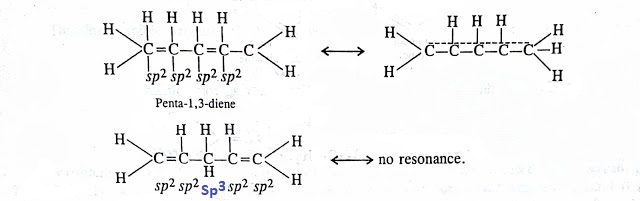
Benzene is reluctant to show addition reaction due to resonances its loses its pure double bond character rendering it partial single bond character to double bond and double bond character to single bond. This causes for reluctance of π-electrons to participate in addition reactions. Moreover addition reaction destroyes the aromaticity of the benzene molecule by affecting π-cloud destruction.
Related Questions: