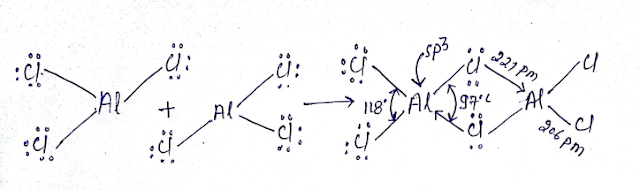
3C-2e BOND OR BANANA BOND:
EXAMPLE FORMATION OF B2H6:
(1) Formation of 3C-2e bond in B2H6 is best explain by MOT and total number of bond in B2H6 is 6 (3C-2e=2 and 3C-4e=4)
(2) Bridge bonds are longer than terminal bond because at bridge bonds electrons are delocalized at three centres
(3) Bond energy (441kj/mole) of B-H-B bond is greater than bond energy (381 K j/mole) of B-H bond.
(4) Hybridization of B atom is sp3, so non planer, and non polar (U=0)
(5) B2H6 Methylated up to B2H2 Me4
(6) B2H6 is hypovalent molecule hence act as Lewis acid and undergoes two type of cleavage when react with Lewis base