Related Questions:
Search This Blog
Saturday, May 30, 2020
Why are bridge head carbocations unstable?
According to Bredt’s rule a bridgehead carbon atom of bicyclo compound cannot be sp2 hybridised or in other word a bridgehead carbon atom cannot be form double bond. unless the ring that contains at least eight atoms.
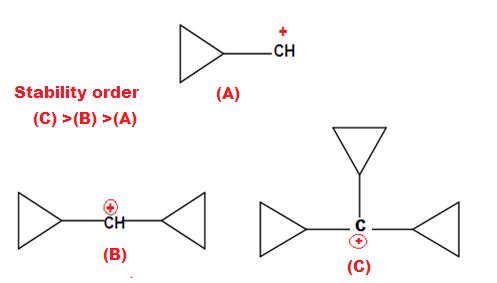
Why is cyclopropyl methyl carbocation exceptionally stable?
The exceptional stability of cyclopropane methyl cation can be explained by the concept of dancing resonance concept. The stability of additional cyclopropyl group , is result of more conjugation between the bent orbital of cyclopropyl ring and cationic carbon.
The most stable carbocation known till date in organic chemistry is explain by Dancing resonance.
Related Questions:
Friday, May 29, 2020
Azabicyclo[2,2,1]heptane is more basic thantriethylamine why?.
Which carbocation is more stable : Benzyl or Tertiary?
Actually answers of this question is always confusing, most of the authors believe benzyl carbocation is more stable than tertiary because benzyl carbocation involves in resonance.
But some of the authors believe that Tertiary carbocation is more stable as it involves maximum +I effect and maximum hyperconjuation +H (9-alpha hydrogens). Maximum +I and +H is more dominant than +M effect. Thus tertiary carbocation is more stable than benzyl carbocation.
Important note:
Stability of Benzyl , allylic and tertiary alkyl carbocation is practically almost same .so that stabilities infact cannot be compared.
Similar Questions:Tuesday, May 26, 2020
100 g of C6H12O6, (aq.) solution has vapour pressure is equal to 40 torr at certain temperature. Vapour pressre d H20(l) is 40.8 torr at same temperature if this solution is cooled to -0.93°C, what mass of ice wil be seperated out ? ( Kf :1.86 kg mol-1)
Subscribe to:
Posts (Atom)