(1)
Each carbon is linked to another atom and there is very closed packing in
structure of Diamond.
(2)
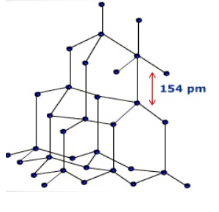
Density and hardness is very much greater for diamond because of closed packing
in diamond due to sp3 hybrid and are tetrahedrally arranged around it. And C-C
distance is 154pm
(3) Diamond has sharp cutting edges that's why
it is employed in cutting of glass.
(4)
Diamond crystals are bad conductor of electricity because of absence of mobile
electron.
(5)
1 carat of diamond = 200 mg.
(6)
Diamond powder if consumed is fatal and causes death in minutes.

No comments:
Post a Comment