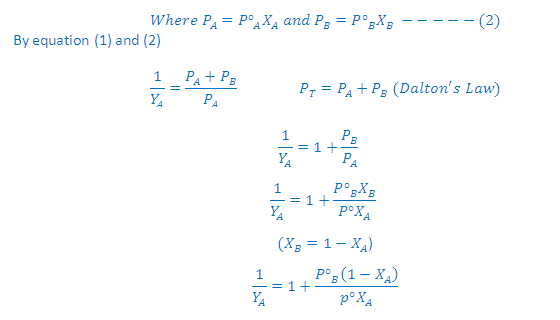An
ideal solution of substance A and B is one in which both substances follow
Raoult’s law for all values of mole fractions. Such solutions occurs when the
substances are chemically similar so that the intermolecular forces between A
and B molecules are similar to those between two A molecules or between two B
molecules. The total vapour pressure over an ideal solution equals the sum of
the partial vapour pressures, each of which is given by Raoult’s law.
(1) Condition for a solution
to be Ideal
Two liquids on mixing form an ideal solution
only when
(1) Both have similar structures and polarity
so that they have similar molecular environment.
(2) Both have similar molecular sizes.
(3) Both have identical intermolecular forces.
(4) The liquid should not dissociate or
associate each other.
(5)
For the solid solute, solution must be extremely diluted.
(2) Characteristic of Ideal Solution:
(3) Examples of Ideal
Solution: Solution of benzene C6H6
and toluene C6H5CH3 are ideal. Note the
similarity in their structural formula.
Suppose a solution is 0.70 mole
fraction in benzene and 0.30 mole fraction in toluene. The vapour pressure of
pure benzene and pure toluene are 75 mmHg and 22 mmHg respectively. Hence the
total vapour pressure is
Other Example of Ideal Solutions
(1)
Benzene + Toluene,
(2) n
hexane + n Heptane;
(3)
Chlorobenzene + Bromobenzene
(4) Ethyl bromide + Ethyl iodide;
(4)
n-Butyl chloride + n-Butyl bromide
(5) Ethyl alcohol + Methyl alcohol
(6) Tetrachloromethane + Tetrachlorosilane
REAL OR NON - IDEAL SOLUTIONS
Those solution which do not obey Raoult’s law
over entire range of composition and deviate from ideal behaviour, are real or
non – ideal solution.
Distinction between Ideal and Non Ideal Solutions:
Types of non-ideal solutions:
(1) Non
ideal solutions showing positive deviation
(2) Non ideal solutions showing negative
deviation.
(1) Non ideal solutions showing positive deviation:
(A)Characters for positive deviation:
When two liquids A and B on mixing form this
type of solution and show following characters
(1)
Non-ideal solution
showing positive deviation from Raoult’s law.
(2) A—B attractive force should be weaker
than A—A and B—B attractive forces.
(3) ‘A’ and ‘B’ have different shape,
size and character.
(4) ‘A’ and ‘B’ escape easily showing
higher vapour pressure than the expected value.
(5) The solution showing positive
deviations from ideal behaviour for those type of solutions,
(B) Condition of positive
deviation:
(C) Graph
of Positive deviation:
(D) Examples and cause of Positive
Deviation:
(A) Difference in
extent of association in two liquids
(1)
H2O and CH3OH (Methanol)
(2)
H2O and ROR’ (Ester)
(3)
H2O and CHCl3 (Chloroform)
Explanation:
Mixture of above pair produces a high distorted curve with maximum vapour pressure.
(B)
Association in one of the liquids through H-bonding
(4)
C2H5OH and C6H6
(Benzene)
(5)
ROH and ROR’ (Ester)
(6)
ROH and CHCl3 (Chloroform)
(7)
ROH CH3COCH3 (Acetone)
(8)
ROH and C6H12 (Cyclohexane)
Explanation:
(C)
Greater difference in length of hydrocarbon part of members of same homologous
series
(9)
n-butane and n-Heptane
(D)
Difference in polarity of
liquids: General Examples are when one is polar and other is non polar
(10)
CCl4 and CHCl3
Explanation:
(5)
Greater Difference in molar mass of non-polar molecules.
(11) CCl4
and C6H6
(13)
CCl4 and Toluene
(14)
Acetone and Benzene
(15)
CS2 and Acetone
(16)
CH3OH and Benzene
Explanation:
(2) Non ideal solutions showing negative deviation
(A) Characters for Negative deviation:
When two liquids
A and B on mixing form this type of solution and shows following character
(1) Non-ideal
solution showing negative deviation from Raoult’s law.
(2) A—B attractive force should be
greater than A—A and B—B attractive forces.
(3) ‘A’ and ‘B’ have different shape,
size and character
(4) Escaping tendency of both components
‘A’ and ‘B ’is lowered showing lower vapour pressure than expected ideally.
(B) Condition of Negative deviation:
The solution showing large negative deviations from ideal
behaviour and the vapour pressure of each component is considerably less than
that predicted by Raoult’s law, for these type of solutions.
(C) Graph
of Negative deviation:
(D) Examples and cause Positive
Deviation:
(1)
An acidic &
a basic liquid: Due to strong intermolecular hydrogen Bonding between
the proton of the acid & lone pair
of the donor atom of the basic liquid (C6H5OH & C6H5NH2)
(2)
Haloalkanes
(like chloroform) with more electronegative atoms: like oxygen or nitrogen or fluorine containing liquid (like ketones, esters, ethers, amines etc) due to
formation of Hydrogen – bonding between these.
Exception:
Excluding ALCOHOLS
which are highly associated and would show positive deviations.
(3)
Aqueous
solutions of strong volatile acids and water: For example sulfuric acid, nitric acid etc., which give non-volatile ions with
water
Newly form hydronium ions and Sulphate ions strongly
associated hence these solution show negative deviation