Determination of composition
in vapour phase:
The composition of the vapour in equilibrium with
the solution can be calculated applying Daltons’ law of partial pressures. Let
the mole fractions of vapours A and B be YA and YB respectively. Let PA
and PB
be the partial pressure of vapours A and B respectively and total pressure PT.
In Vapours phase:
YA=
mole fraction of A in vapour phase
YB
= mole fraction of B in vapour phase
(YA+YB =1)
In liquid solution phase:
XA
= mole fraction of A in liquid phase
XB
= mole fraction of B in liquid phase
(XA + XB = 1)
According to Raoult’s Law: The partial
pressure of any volatile component of a solution at any temperature is equal to
the vapour pressure of the pure component multiplied by the mole fraction of
that component in the solution.
Where
XA and XB is the mole fraction of the component A and B in
liquid phase respectively
According to Dalton’s Law:
The vapour behaves like an ideal gas, then according to Dalton’s law of partial
pressures, the total pressure PT is given by:
Partial
pressure of the gas = Total pressure x Mole fraction
PA = PT YA
and PB =PT YB
Where YA and YB is the
mole fraction of the component A and B in gas phase respectively
Combination of Raoult’s and Dalton’s Law:
(3) Thus, in case of ideal solution the vapour
phase is phase is richer with more volatile component i.e., the one having
relatively greater vapour pressure
Graph Between 1/YA Vs 1/XA:
According
to Dalton’s law of partial pressures, the total
pressure PT is given by:
Partial
pressure of the gas = Total pressure x Mole fraction
Where YA and YB is the
mole fraction of the component A and B in gas phase respectively
According
to Raoult’s law:
On rearrangement of this
equation we get a straight line equation:







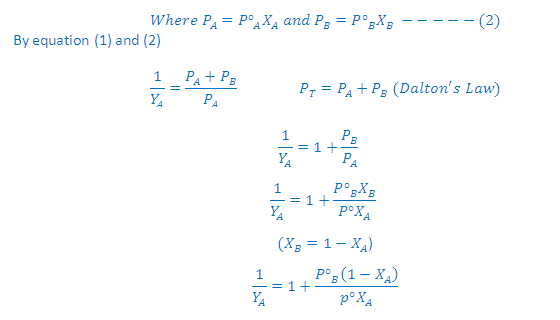

I honestly appreciate the blog you have posted. Really looking forward to this kind of words.
ReplyDeletegreat
ReplyDelete