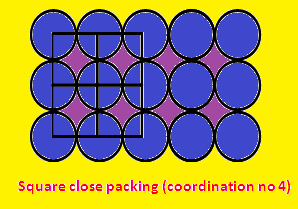
(2) Take two 2D square close packing
sheet and Placing a second square packing layer (sheet) directly over a first
square packing layer forms a "simple cubic" structure.
(3) The simple “cube” appearance of the
resulting unit cell is the basis for the name of this three dimensional
structure.
(4) This packing arrangement is often symbolized as
"AA...", the letters refer to the repeating order of the
layers, starting with the bottom layer.
(5) The coordination number of each
lattice point is six. This becomes apparent when inspecting part of an adjacent
unit cell.
(6) The unit cell contain eight corner spheres, however, the total number of
spheres within the unit cell is 1 (only 1/8th of each sphere is actually inside
the unit cell). The remaining 7/8ths of each corner sphere resides in 7
adjacent unit cells.
(7) PACKING EFFICIENCY):
In simple cubic unit cell:
(1) Let ‘a’ be the edge length of the unit cell and r be
the radius of sphere.
(2) As sphere are touching each other therefore a = 2r
(3) No. of spheres per unit cell = 8*1/8=1
8*1/8=1
(4) Volume of the sphere = 4/3(pi) r3
(5) Volume of the cube = a3= (2r)3
= 8r3
(6) Packing efficiency (space occupied):
(7) Density of simple unit cell:
(8) Coordination Number:
(1) The nearest neighbour distance is just the lattice parameter
(a) therefore
coordination number for a given atom in SCC unit cell is 6 (six).
(2)
The next nearest
neighbour are 12 at distance a/root 2 (each face diagonal in x ,y and Z plane).
(3) 3rd neighbour (Next to Next nearest
neighbour) are (8)
at distance a root 3 (each corner along body diagonal.




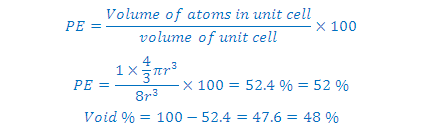





No comments:
Post a Comment