Total energy of (E) of an electron
revolving in nth orbit is equal to sum of kinetic
energy and Potential energy.
We know the
electron revolve around nucleus due balancing of two forces columbic and
centrifugal forces
This is the
famous Bohr’s equation applicable to Hydrogen like atoms or ions as He+1,
Li+2 , Be+3
etc.
The factor
(4 pi epsilon zero) is known as permittivity factor and its numerical value is
1.11268*10-10C2N-1M-2 ( In CGS Unit K= 1)
Pi= 22/7=
3.424, me=9.109 *10-31 kg, e = 1.602 *10-10 C and h=
6.626*10-34 j-s
Calculation of En
in SI Unit:
Bohr’s energy in
electron volt:
We know that,
1eV = 1.602 *10-19 J hence
Energy in term of Rydberg’s
Constant:
Relation between Total
energy (TE), Kinetic energy (KE) and Potential energy (PE):
Important conclusions:
(1) The minus sign for the energy of an electron
in an orbit represents attraction between the +vely charged nucleus and negatively charged electron.
(2) Energy of an electron at infinite distance
from the nucleus is zero.
(3) As an electron approaches the nucleus, the
electrical attraction increases, energy of electron decreases and it becomes
negative.
(4) Energy of an electron increases as the value
of ‘n’
increases i.e.
(5) Value
of ‘n’
remaining unchanged, the amount of energy associated with an electron remains
unaltered.
(6) Energy of electron in first, second, third and fourth orbit are –13.6, –3.4, –1.5, and
–0.85 eV/atom respectively.
(7) Although the energy of electron increases with
increase in the value of ‘n’ (orbit), yet the difference of energy between
successive orbits decreases. Thus E2 – E1 > E3 – E2
> E4 – E3 > E5 – E4 >,
etc….




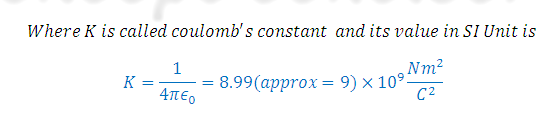






df
ReplyDelete