SN2-Ar Mechanism:
Nucleophilic
aromatic substitution takes place by a two-step reaction known as an SN2-Ar (SN2 –Aromatic bimolecular.) reaction.
The SN2-Ar mechanism and involves a resonance-stabilized anionic
intermediate called the Meisenheimer complex.
ILLUSTRATIVE EXAMPLE:
Meisenheimer complex
Intermediate:
Step-(1): An Addition
Step-(2): An Elimination:
Thus, the overall mechanism is an Addition–Elimination Mechanism. the
Meisenheimer complex is stable enough to form only if an electron-withdrawing
group therein can stabilize
the negative charge by resonance.
Characteristics of SN2-Ar
reaction:
(1) It
is a Nucleophilic aromatic Substitution reaction by Addition–Elimination Mechanism.
(2)
Rate of this reaction depends upon concentration of halide and nucleophile both
(3)
Order of reaction is (2) Bimolecular reaction.
(4)
Carbanion is intermediate. Hence rearrangement not possible.
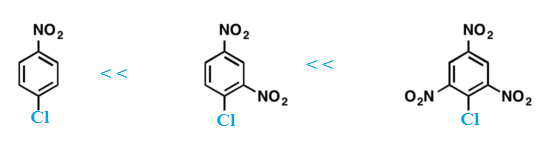
(5) Rate of reaction increase on increasing number of EWG
which stabilized Meisenheimer complex.
(6)
Rate of fluorine derivative (fluoro benzene) is most reactive because of high (–I)
inductive effect
.
(7) Only those
halogen replace which are ortho or para position with compared to nitro group.
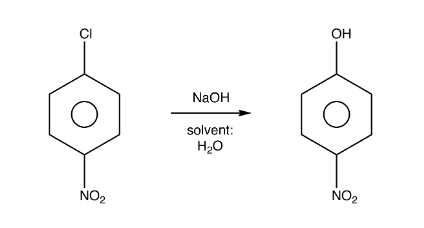
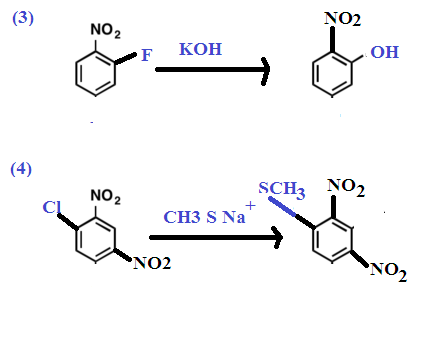
Other Examples:
(3) Substitution of unactivated hydrogen: (Benzyne Mechanism):







No comments:
Post a Comment