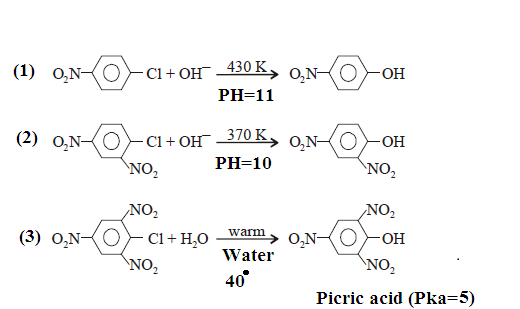
SN2 (Aromatic)
Type: Chloro benzene
does not under goes Nucleophilic
substitution reaction in ordinary condition due to following reason.
C–X bond in
aryl halide is stable due to delocalisation of electrons by resonance. Also
(C–X) bond possesses a double bond character like vinyl chloride and is
stronger than C–X bond in alkyl halide.
Case-(1): Hence, SN reaction is not possible
in benzene nucleus under ordinary conditions. However, under high temperature
and pressure, SN reaction is made possible.
Case-(2): However Nucleophilic substitutions occur
in the presence of one or more electron withdrawing (EWG) at ortho and para position.
These electron-withdrawing groups must be positioned ortho or para to the
leaving group. The greater the number of electron-withdrawing substituents, the
easier it will be to carry out the nucleophilic aromatic substitution
reactions.
EXAMPLE:
While electron releasing
group (ERG) destabilizes carbanium ion and deactivated.
OTHER EXAMPLES:








No comments:
Post a Comment