SN-Ar : More general attack of nucleophile on
unsubstituted benzene ring is very difficult as compared to attack by Electrophile
hence nucleophilic substitution of hydrogen from benzene is also difficult
because..
(1) The pi electron
cloud of benzene nucleus repels the approaching nucleophile.
(2) It is
difficult to accumulate negative charge (electron) donated by attacking
nucleophile.
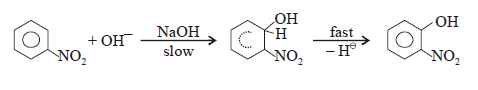
However in
the presence of one the electron withdrawing group like nitro group (-NO2)
on benzene ring, then it is sufficiently activate ortho and para position
ILLUSTRATIVE EXAMPLE:
Nitro phenol give ortho nitro phenol and also little amount of para nitro phenol on treatment with
strong NaOH
in the presence of oxidizing agents like KNO3 and K3 [Fe(CN)6].
MECHANISM: (SN-Ar):
Important note : Since OH- is better leaving group than hydride ion (H- )
but the presence of oxidizing agents like
air , KNO3 and K3 [Fe(CN)6]
which encourage the elimination of hydride ion.
Other Examples:

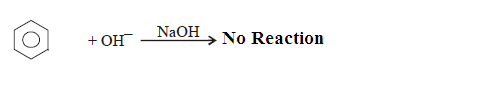


No comments:
Post a Comment