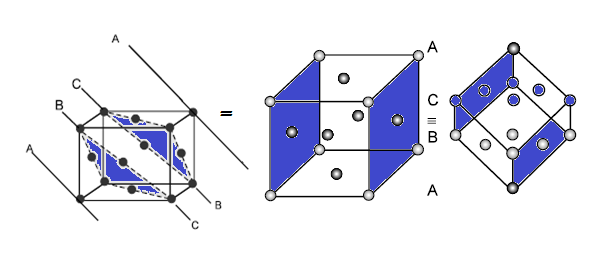
CCP or FCC has two lattice point corner as well as face centred:
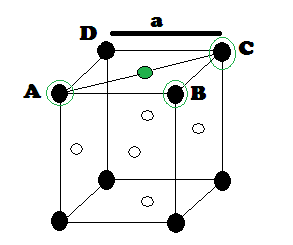
Suppose 'r' be the radius of sphere and 'a' be the edge length of the cube As there are 4 sphere in FCC unit cell
(1) Relation between radius (r) and side (a)
In FCC, the corner spheres are in touch with the face centred sphere. Therefore, face diagonal AD is equal to four times the radius of sphere AC = 4r
But from the right angled triangle ABC:
This is the relation between radius (r) and edge length (a)
So we can find the edge length in term of radius is
(a) =2√2 r
Edge uncovered can be calculated as :
2√2 r- 2r = 0.83 r
Percentage edge uncovered : (0.83r/a)×100=29.27 %



No comments:
Post a Comment