SOLUTION:
Here, we are given that
Ea = 22.5 kcal mol-1 = 22500 cal mol-1
T = 40°C = 40 + 273 = 313 K
k = 1.8 × 10-5 sec-1
Substituting the values in the equation
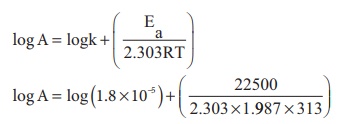
log A = log (1.8) −5 + (15.7089)
log A = 10.9642
A = antilog (10.9642)
A = 9.208 × 101^0 collisions s^−1

No comments:
Post a Comment