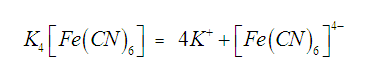Coordination
compounds are those molecular compounds which retain their identities even when
dissolved in water or any other solvent and their properties are completely
different from those of the constituents.
The extent
of dissociation of a complex ions into its constituents is decided by a
constant which is called formation constant of the complex. The constituents
obtained may or may not be detected practically which depends on concentration
and the reagents.
For example, potassium ferrocyanide is a
complex compound. It is formed by adding KCN to a saturated solution of ferrous
cyanide. If we add a solution of potassium
cyanide to a white precipitate of nickel cyanide, Ni (CN)2, the
precipitate immediately dissolves and a red orange solution of a new
compound is obtained.
When potassium
ferrocyanide is dissolved in water, it does not give the usual tests for Fe2+
and CN– ions indicating that these ions which were originally
present are not formed when potassium ferrocyanide is dissolved in water, Actually these ions are present in the
form of a new ion, called ferrocyanide
ion which is a complex ion and does
not ionize into constituent ions.
Compounds
containing complex ions are called complex
compounds. Since the complex ions have coordinate1bonds in their
structures, these are also known as coordinate ions and hence the corresponding
compound as coordinate compound.
Other common complex ions are nickel
cyanide, [Ni(CN)4]-2,
cupper ammonium, [Cu(NH3)4]2+
argentocyanide,[Ag(CN)2]+
Thus complex ion may be defined as an
electrically charged (cationic or anionic) or even a neutral species and is
formed by the combination of a simple cation with more than one neutral
molecule or negative ion. For example, ferrocyanide ion is formed by the union
of six cyanide ions with ferrous ion; [Ag(NH3)2]+
is formed by the combination of two moles of ammonia and one mole of Ag+
ion. The anions or neutral molecules attached to the central metal atom are called
ligands. The central metal cation is
generally a transition metal and has a positive