(1) In Graphite Carbons
are sp2 hybridised out of the four valence electrons, three involved in (sp2-sigma) covalent
bonds form hexagonal layers and fourth unhybridised p– electron of each carbon
forms an extended delocalized p-bonding with carbon
atoms of adjacent layers
(2) Each carbon is
linked with 3 carbons and one carbon will be left and form a two dimensional
shed like structure.
(3)
Distance between two layers is very large so no regular bond is formed between
two layers. The layers are attached with weak vander waal force of attraction.
(4)
The carbon have unpaired electron so graphite is a good conductor of current.
(5) The C-C bond length
within a layer is 141.5 pm while the inter layer
distance is 335.4 pm shorter than that of Diamond (1.54 Å).
(6) Due to wide
separation and weak interlayer bonds, graphite is sift , greasy and a lubricant
character and low density.
(7) Graphite marks the
paper black so it is called black lead or plumbago and so it is used in pencil
lead.
(8) Composition of
pencil lead is graphite plus clay .the percentage of lead in pencil is zero .
(9) Graphite has high melting point so it is
employed in manufacture of crucible.
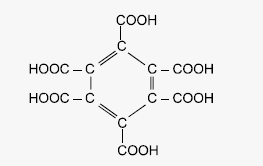
(10) Graphite when heated
with oxidizing agents like alkaline KMnO4 forms mellatic
acid
(Benzene hexa carboxylic acid).
(11) Graphite on
oxidation with HNO3 gives acid i.e. known as Graphite acid C12H6O12