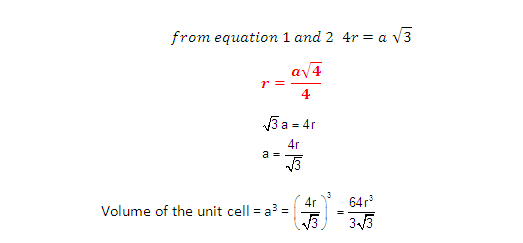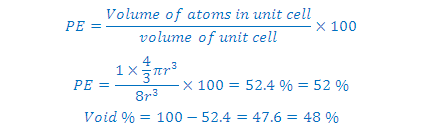Packing
sphere in any pattern in any fashion generate some empty space between them
these space (gaps) are known as voids they are named according to the
orientation of spheres constituting voids.
(A) VOID IN TWO DIMENSIONS:
(1) Triangular Void: The triangular voids are found in the planes of
the close packed structures, whenever three spheres are in contact in such a
fashion. Coordination number of trigonal void is three.
Where, R=
Radius of the sphere, r = maximum radius of a sphere that can be placed
inside the void.
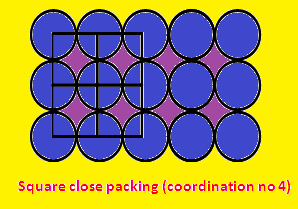
(2) Square void: The square voids are found in the
planes of the close packed structures, whenever three spheres are in contact in
such a fashion. The coordination number of square void is four.
(B) VOIDS IN THREE DIMENSIONS:
(3) Cubical void: The cubical void is generally not found in closed
packed structures, but is generated as a result of distortions arising from the
occupancy of voids by larger particles. Cubical voids found in simple cubic
unit cell and it is 3-dimensional and has coordination number eight (8).
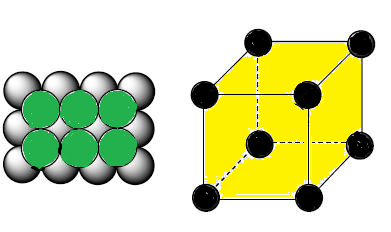
(C) VOIDS IN CLOSE PACKING IN 3D (HCP AND CCP):
In hcp as well as ccp only 74% of the
available space is occupied by spheres. The remaining space is vacant and
constitutes interstitial voids or interstices or holes. These are of two types
(1) Tetrahedral voids (2) Octahedral voids
(1) Tetrahedral voids: In close packing arrangement, each
sphere in the second layer rests on the hollow (triangular void) in three
touching spheres in the first layer. The centres of theses four spheres are at
the corners of a regular tetrahedral. The vacant space between these four
touching spheres is called tetrahedral void. In a close packing, the number of
tetrahedral void is double the number of spheres, so there are two tetrahedral
voids for each sphere
Radius of
the tetrahedral void relative to the radius of the sphere is 0.225
In
a multi layered close packed structure , there is a tetrahedral
hole above and below each atom hence there is twice as many tetrahedral holes
as there are in close packed atoms.
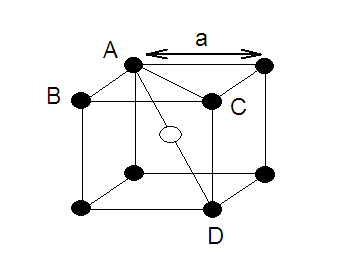
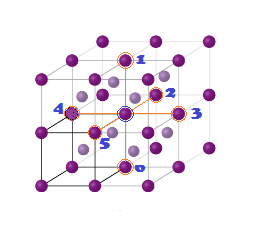
DERIVATION OF RELATION: Derivation of the relationship between the radius (r) of the octahedral
void and the radius (R) of the atoms in close packing.
A sphere into the octahedral void is shown in the
diagram. A sphere above and a sphere below this small sphere have not been
shown in the figure. ABC is a right angled triangle. The centre of void is A.
(2) Octahedral voids: As already discussed the spheres in the second
layer rest on the triangular voids in the first layer. However, one half of the
triangular voids in the first layer are occupied by spheres in the second layer
while the other half remains unoccupied. The triangular voids ‘b’ in the first
layer are overlapped by the triangular voids in the second layer. The interstitial
void, formed by combination of two triangular voids of the first and second
layer is called octahedral void because this is enclosed between six spheres
centres of which occupy corners of a regular octahedron
In
close packing, the number of octahedral voids is equal to the number of
spheres. Thus, there is only one octahedral void associated with each sphere.
Radius of the octahedral void in relation to the radius of the sphere is 0.414
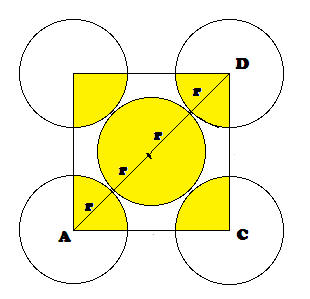
DERIVATION OF RELATION: Derivation of the relationship between radius (r) of the tetrahedral void
and the radius (R) of the atoms in close packing: To simplify calculations, a
tetrahedral void may be represented in a cube as shown in the figure. In which
there spheres form the triangular base, the fourth lies at the top and the
sphere occupies the tetrahedral void.
RADIUS
RATIO RULES IN IONIC SOLIDS:
The
structure of many ionic solids can be accounted by considering the relative
sizes of the cation and anion, and their relative numbers. By simple
calculations, we can work out as how
Many ions
of a given size can be in contact with a smaller ion. Thus, we can predict the coordination
number from the relative size of the ions.
Following conditions must be satisfied simultaneously during the stacking
of ions of different sizes in an ionic crystal:
(1) An anion and a cation are assumed to be hard spheres always touching
each other.
(2) Anions generally will not touch but may be close enough to be in
contact with one another in a limiting situation.
(3) A cation should surround itself with as many anions as possible. Each
ion tends to surround itself with as many ions of opposite sign as possible to
reduce the potential energy. This tendency promotes the formation of
close-packed structures.
RADIUS
RATIO AND COORDINATION NUMBER:
LOCATING TETRAHEDRAL AND OCTAHEDRAL VOIDS:
(1) The close packed structures have both
octahedral and tetrahedral voids. In a ccp structure, there is 1 octahedral
void in the centre of the body and 12 octahedral void on the edges. Each one of
which is common to four other unit cells. Thus, in cubic close packed
structure.
Octahedral voids in the centre of the cube =1
Effective number of octahedral voids located
at the 12 edge of = 12*1/4=3
Total number of octahedral voids = 4
(2) In ccp structure, there are 8
tetrahedral voids. In close packed structure, there are eight spheres in the corners
of the unit cell and each sphere is in contact with three groups giving rise to
eight tetrahedral voids
TETRAHEDRAL VOIDS LOCATION:
(3) Circles labelled T represent the centers of the tetrahedral interstices in the ccp
arrangement of anions. The unit cell "owns" 8 tetrahedral
sites.
OCTAHEDRAL VOIDS LOCATION:
Circles labelled O represent centers of the octahedral interstices in the ccp
arrangement of anions (FCC unit cell). The cell "owns" 4
octahedral sites.