Inversion
of amines takes place at room temperature and required 64 k j per mole energy.
An amine
such as ethyl methyl amine has a tetrahedral (sp3) nitrogen atom, and the
nitrogen lone pair in an sp3 orbital.
However this tetrahedral amine structure is not static. It is found that the
nitrogen atom inverts. The lone pair disappears from one face, moves through
the nucleus, and reappears on the opposite face. The lone pair repels the ethyl
group, the hydrogen atom, and the methyl group, so as the lone pair reappears,
these groups move away. As the lone pair is tunneling through the nucleus, the
nitrogen has just three attachments so this nitrogen atom is trigonal planar
with sp2 hybridization. Overall
flipping of amines is called as inversion of Amine. This magical act is also known
as quantum mechanical
tunneling.

The
tetrahedral amine structures are in equilibrium. Furthermore, these structures are
enantiomers. All physical properties of enantiomers are identical except for
the direction in which they rotate plane polarized light). From this we can
conclude that the two enantiomeric amines are present in equal amounts and have
equal stability .
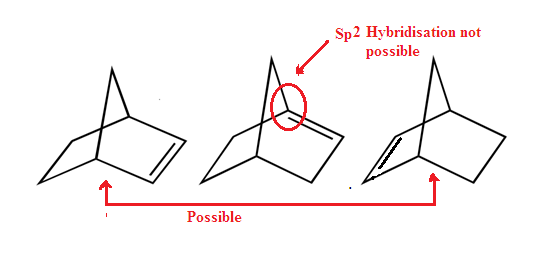
There are
two conditions in which a inversion of nitrogen atom does not take place.
1: No Lone Pair:
2: Ring Strain:
When the
amine nitrogen is not part of a ring, this bond angle change is easily and inversion
takes place. However, if the nitrogen atom is part of a three-membered or a
four-membered ring then inversion is significantly retarded by strain.
Effect of Inversion on basicity of amines:
The rate
of nitrogen inversion also correlates with, among other things, hybridzation.
But in cases where these "other things", such as ring constraints,
limit nitrogen inversion, then the rate of nitrogen inversion may not correlate
with hybridization. So the rate of nitrogen inversion does not always correlate
with hybridization and is therefore not a good indicator of lone pair
"availability".
It is clear
that basicity of amines reduced by inversion of amines.
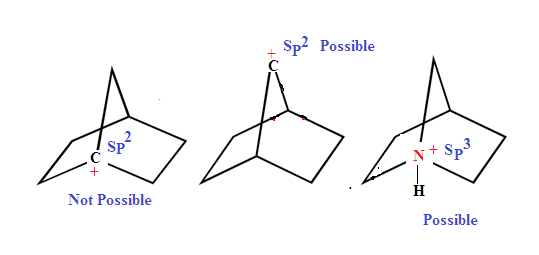
ILLUSTRATIVE EXAMPLE (1): 1-Azabicyclo[2,2,1]heptane
is more basic than triethylamine why?.
SOLUTION: 1-Azabicyclo[2,2,1]heptane
is more basic than triethylamine . Because in case of triethylamine the
lone pair of electrons is less available in the latter due to rapid nitrogen
inversion. Nitrogen inversion is not possible in the bicyclic amine.
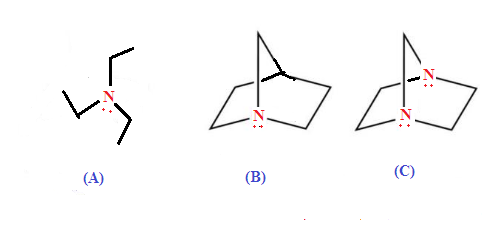
ILLUSTRATIVE EXAMPLE (2): Which
of the following compound is more basic?
SOLUTION: Option (A) is
least basic because Option (B) and (C) both have restriction of inversion hence more
basic while option (C) is least basic than option (B) due to –I effect
of N-atom to another reduce availability of lone pair of nitrogen atom.
ILLUSTRATIVE EXAMPLE (3):The correct order of decreasing basicity of the compounds is :
SOLUTION: III > II > IV > I
In Case of Option (III) their is restriction of inversion take place at bridge head nitrogen atom hence it is most basic than (II), while (II) is more basic then (VI) because its lp does not delocalized while lp of (IV) is delocalized with ring hence less basic but (I) is least basic to all due to presence of three strong -I group.