Search This Blog
Sunday, May 24, 2020
B-F bond length in BF3 is shorter than B-F bond length in (BF4)- why?
B-F bond length increases when BF3(130 pm) reacts with F- to form (BF4)- [143 pm]. Its due to absence of Back-bonding in (BF4)- hence B-F bond has completely single bond character
Dipole moment of PCl3F2 and P(CH3)3(CF)2 molecules are zero while dipole moment of PCl2F3 and P(CH3)2(CF3)3 are non zero why?
According to bent rule more electronegative atom or group attached those hybrids orbital have minimum S- character.
There is in trigonal bipiramidal (TBP) Geometry we known that axial orbital hare no s-character so F and -CF3 group are attached with equatorial positions. Hence dipole moment of P(CH3)2(CF3)2 is zero. While in case of PCl2F3 and P(CH3)2(CF)3 molecules one of the F and –CF3 group are also present at equatorial position hence there is net dipole moment.
Related Questions:
What is Bent’s
rule of hybridization?
Which of the
following compound have longest (S=O)bond length , O=SF2, O=SCl2, O=SBr2.
Why Bond length
of O-O is greater in H2O2 than O2F2?
How to arrange
increasing (C-H) bond length in increasing order and H-C-F bond angle in the
given compounds, CH4, CH3F, CH2F2 and CHF3 ?
Dipole moment of
PCl3F2 molecule is zero while dipole moment of PCl2F3 molecule is non zero why?
Dipole moment of
P(CH3)2(CF3)2 molecule is zero while dipole moment of P(CH3)2(CF)3 molecule is
non zero why?
Dipole moment of PCl2F3 is non zero while dipole moment of PCl3F2 is zero why?
According to bent rule more electronegative atom or group attached those orbital have minimum S- character. There is in Trigonal bipiramidal (TBP) Geometry we known that axial orbital hare no S- character so F atom attached with axial positions only. Hence PCl3F2 has zero dipole moment.
What is Banana bond (3C-2e bridge bond) ? Explaine with suitable examples.
3C-2e BOND OR BANANA BOND:
EXAMPLE FORMATION OF B2H6:
(1) Formation of 3C-2e bond in B2H6 is best explain by MOT and total number of bond in B2H6 is 6 (3C-2e=2 and 3C-4e=4)
(2) Bridge bonds are longer than terminal bond because at bridge bonds electrons are delocalized at three centres
(3) Bond energy (441kj/mole) of B-H-B bond is greater than bond energy (381 K j/mole) of B-H bond.
(4) Hybridization of B atom is sp3, so non planer, and non polar (U=0)
(5) B2H6 Methylated up to B2H2 Me4
(6) B2H6 is hypovalent molecule hence act as Lewis acid and undergoes two type of cleavage when react with Lewis base
What is bridge bond ? explaine 3C-4e bridge bond with suitable examples .
3C-4e BOND or 3C-4e BRIDGE BOND:
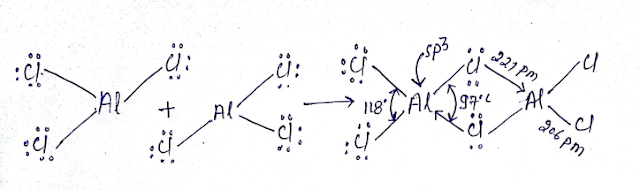
Al2Cl6 Dimmerised by 3C-4e bond bridge bond:
Al2Cl6 is neither hypovalent nor hypovalent rather its octet is complete. We will used MOT here it cannot act as Lewis acid due to crowding in spite having vacant d orbital’s however Alcl3 act as Lewis acid.
Al2Cl6 contains six bond having two bridge bond(3c-4e) and four bond is (2C-2e)
Boron do not formed bridge bond because boron experience steric crowding.
Topic:
Bridge Bonding,
CHEMICAL BONDING:
Subscribe to:
Comments (Atom)