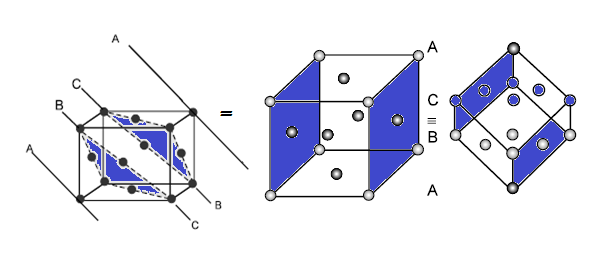
Effect of temperature on crystal structure:
Increase of temperature decreases the coordination of number, e.g. upon heating to
760 K, the CsCl type crystal structure having coordination 8:8 changed to NaCl type crystal structures having coordination 6:6.
760 K, the CsCl type crystal structure having coordination 8:8 changed to NaCl type crystal structures having coordination 6:6.
Effect of pressure on crystal structure:
Increase of pressure increases the Co – ordination number during crystallization e.g. by applying pressure, the NaCl type crystal structure having 6:6 coordination number changes to CsCl type crystal having coordination number 8:8
Fore more details click ⏬
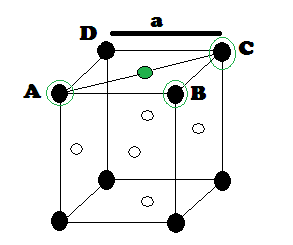
CAESIUM CHLORIDE (CsCl) STRUCTURE: