Search This Blog
Tuesday, June 9, 2020
Sunday, June 7, 2020
The vapour pressure of two pure liquids A and B, that form an ideal solution are 100 and 900 torr respectively at temperature T. This liquid solution of A and B is composed of 1 mole of A and 1 mole of B. What will be the pressure, when 1 mole of mixrure has been vaporized?
Saturday, June 6, 2020
What is the effect of temperature and pressure on crystal structure of Caesium Chloride?
Effect of temperature on crystal structure:
Increase of temperature decreases the coordination of number, e.g. upon heating to
760 K, the CsCl type crystal structure having coordination 8:8 changed to NaCl type crystal structures having coordination 6:6.
760 K, the CsCl type crystal structure having coordination 8:8 changed to NaCl type crystal structures having coordination 6:6.
Effect of pressure on crystal structure:
Increase of pressure increases the Co – ordination number during crystallization e.g. by applying pressure, the NaCl type crystal structure having 6:6 coordination number changes to CsCl type crystal having coordination number 8:8
Fore more details click ⏬
CAESIUM CHLORIDE (CsCl) STRUCTURE:
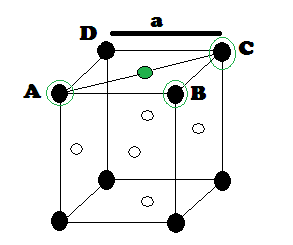
What fraction of edge is not covered by atoms in FCC unit cell ?
CCP or FCC has two lattice point corner as well as face centred:
Suppose 'r' be the radius of sphere and 'a' be the edge length of the cube As there are 4 sphere in FCC unit cell
(1) Relation between radius (r) and side (a)
In FCC, the corner spheres are in touch with the face centred sphere. Therefore, face diagonal AD is equal to four times the radius of sphere AC = 4r
But from the right angled triangle ABC:
This is the relation between radius (r) and edge length (a)
So we can find the edge length in term of radius is
(a) =2√2 r
Edge uncovered can be calculated as :
2√2 r- 2r = 0.83 r
Percentage edge uncovered : (0.83r/a)×100=29.27 %
What is the number of atoms on one unit cell of HCP?
ANALYSIS OF HCP UNIT CELL:
Number of effective atoms in HCP unit cell (Z):
Lattice point: corner- total 12 carbon contribute 1/6 to the unit cell
Lattice point: face- total face 2 contribute ½
Lattice point: body centre- total atom 3 (100% contribution)
Subscribe to:
Comments (Atom)