Generally neutral atom donates only one lone pair to back bonding because after that, electron density on that atom itself highly reduced but the compound [(CF3)2-Al-O-Al-(CF3)2] is a rare example of two lone pair donation.
Search This Blog
Thursday, December 24, 2020
What is structure (CF3)2-Al-O-Al-(CF3)2 and also find the number of pi bond or Back bond?
What are the structure of (1) N(SiH3)3 (2) {(Me)3Si}2N-Si(Me)3 (3) HN(SiH3)2 and give the Answers of following questions?
Q (1):
which is greater x or y?
Q (2):
which is greater x or z?
Q (3):
which have greater extent of back Bonding?
SOLUTION:
Ans: (1): ‘y’ is greater than ‘x’ because of steric repulsion
of -CH3 group.
Ans: (2): “z’ will be greater because one lone pair going two
places.
Ans; (3): the extant of back bonding is 3rd >1st > 2nd
Which of the following is correct order of (B-O) bond length of following compounds? (1) B(OH)3 (2) B(OMe)3 and (3) B(Me)2OH
SOLUTION:
Extent Back Bonding is 3>1>2
and bond length order is y>x>z
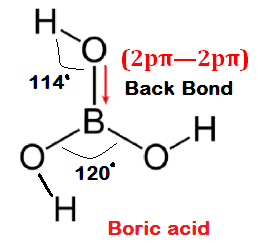
Which of the following correct statement about structure of Ortho boric acid (H3BO3 ) is/are? Statements are as
(1) Angle O-B-O =120
(2) Angle H-O-B >109
(3) Hybridization of atom O close to sp2 and
(4) Molecule is non planer and non polar
Answer: Statement (4) is wrong because molecule is planer and polar
Tuesday, December 22, 2020
What is the structure of Basic beryllium Nitrate (BBN)?
Basic Beryllium nitrate is a Covalent compound and has as unique structure like [Be4O(NO3)], containg six nitrate group. The more interesting structure of Basic Beryllium nitrate because the nitrate group act as indented ligand in forming a bridge between two Be atoms.