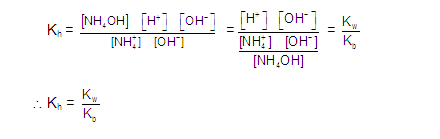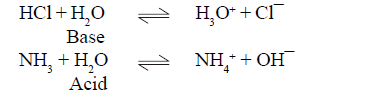Search This Blog
Wednesday, October 31, 2018
[2] CATIONIC SALT HYDROLYSIS:
[1] ANIONIC SALT HYDROLYSIS:
(2) ALKALINE SALTS:(Salts of weak acids and strong bases)
(3) ACIDIC SALTS:(Salts of weak bases and strong acids)
(4) Salts of weak acids and weak bases
(1) ANIONIC HYDROLYSIS OR ALKALINE SALTS HYDROLYSIS:
(Salt of a Weak Acid and Strong Base)
SALTS AND SALT HYDROLYSIS
(2) Salts may taste salty, bitter, a
stringer or sweet or tasteless
(3) Solution of salts may be acidic,
basic or neutral.
(4) Fused salts and their aqueous
solutions conduct electricity and undergo electrolysis
(5)The salts are generally
crystalline solids
CLASSIFICATION
OF SALTS:
These salts
may be classified into four categories.
(A)
SIMPLE SALTS:
The salts
formed by the neutralization process between acid and base. These are of three
types.
(1)
Normal salt:
The salt
formed by the loss of all possible protons (replaceable H+ ions)
For example
NaCl, NaNO3, K2SO4, Ca3 (PO4)2,
Na3BO3, Na2HPO, NaH2PO2 etc.
(2)
Acidic salts:
Salts formed
by incomplete neutralization of polybasic acid. Such salts contain one or more
replaceable H atom.
For examples
NaHCO3, NaHSO4, NaH2PO4, Na2HPO4 etc.
Above salts
when neutralised by base form normal salts.
(3)
Basic salts:
Salts formed
by incomplete neutralization of poly acidic bases are called basic salts. These
salt contain one or more hydroxyl groups.basic salt
when neutralised by acids form normal salts.
Ex.
Zn(OH)Cl, Mg(OH)Cl, Fe(OH)2Cl, Bi(OH)2 etc.
(B)
DOUBLE SALTS:
(1)The
addition compounds formed by the combination of two simple salts are termed as
double salts.
(2) Double
salts are stable in solid state only.
(3) When
dissolved in water, it furnishes all the ions present in the simple salt form
which it has been constituted.
(4)The
solution of double salt shows the properties of the samples salts from which it
has been constituted
For examples
Mohar’s
salt-FeSO4 (NH4)2SO4 .6H2O
(Ferrous ammonium sulphate)
Alum’s- K2SO4Al2 (SO4)3.
24H2O (Potassium ammonium sulphate)
Karnalite- KCl.MgCl2.6 (H2O)
Dolomite- CaCO3.MgCO3 or
CaMg (CO3)2
(C)
COMPLEX SALTS:
(1) Complex
salts are formed by combination of two simple salts or molecular compounds.
For
examples K4Fe (CN)6, Co(NH3)6 SO4 etc.
(2) Complex
salts are stable in solid states as well as solutions
(3) Complex
salts On dissolving in water, if furnishes a complex ion.
(4) The
properties of the solution are different from the properties of the substance
from which it has been constituted.
(D)
MIXED SALTS:
(1)The salt
which furnishes more than one cation or more than one anion when dissolved in
water is Called mixed salt.
FOR EXAMPLE - CaOCl2, NaKSO4, NaNH4HPO4 etc.
SALT
HYDROLYSIS:
[1]
ANIONIC SALT HYDROLYSIS:
[2]
CATIONIC SALT HYDROLYSIS:
[3]
CATIONIC AS WELL AS ANIONIC HYDROLYSIS:
Tuesday, October 30, 2018
BUFFER SOLUTION
(1) DEFINITION
(2)TYPE OF BUFFER SOLUTION
(3) pH OF ACIDIC BUFFER
(4) pH OF BASIC BUFFER
(5) DILUTION OF BUFFER
(6) BUFFER CAPACITY OR BUFFER INDEX
(7) IDEAL BUFFER SOLUTION
The aqueous electrolyte solution which resist the any change in pH even after addition of small amount of strong acid or strong base called buffer Solution.
(1) Simple Buffer Solution or Neutral buffer
(2) Mixed Buffer Solution
Pkb(NH4OH) is 5 and a buffer solution contains 0.1M NH4OH and 0.1M (NH4)SO4 calculate pH of this buffer solution ?
(Ans-5.3 )
Pls of HX is 4.7 , (1) find the pH of Solution having 0.5 M HX and KX 0.25M ? (2) pH of this solution if it is diluted 10000 times ?
(Ans- (1) 4.4 (2) )
Calculate pH of acidic buffer mixture containing 1.0 M HA (Ka=1.5x10-1) and 0.1M NaH .
(Ans- 0.824)
Calculate the mass of NH3 and NH4Cl required to prepared a buffer solution of pH =9.0 when total concentration of buffering reagents is 0.6 mole /litre .( Pkb for NH3 is 4.7, log2 is 0.3010)
(Ans- a=0.2 mole ,b=0.4 mole)
One liter buffer solution is prepared by mixing of 1.0 mole HCOOH (formic acid) and 1 mole HCOONa . (Given Pka HCOOH =4.0) then calculate
(i) pH of buffer
(ii) pH of Solution if 1/3 mole of HCl is added
(iii) pH of Solution if 1/3 mole of NaCl is added
(iv) pH of Solution if it diluted to 10 liter
(v) pH of Solution if it diluted to 1000 liter
(Ans- i-4.0 , ii- 3.7 , iii-4.3010 , vi- 4.0 v- 4.07)
Calculate the pH of an aqueous solution originally containing 0.4 M acetic acid and 0.2 M sodium acetate (ka CH3COOH= 1.8x10-5 ).
(Ans- 4.4)
Calculate pH of Solution Originally having 0.2 M (NH4)SO4 and 0.4 M NH4OH (given Kb NH4OH =1.8x10-5)
(Ans- 9.26)
ILLUSTRATIVE EXAMPLE (8):
In 100 ml of 0.4M C6H6COOH Solution,0.1M C6H6COONa is added , calculate pH of resulting solution (given Ka C6H6COOH is 4x10-5)
(Ans-4.1)
A solution contains 0.2 mole acetic acid and 0.10 mole of sodium acetate ,made up to 10 liter volume , calculate the pH of Solution ( given Ka CH3COOH is 1.8x10-5)
(Ans-4.44)
ILLUSTRATIVE EXAMPLE (10):
What mass of , in gram ,of NaNO2 must added to 700 ml of 0.165 M HNO2 to produce a Solution with pH of 3.50 ? ( Ka HNO2 is 6.0x10-4)
(Ans- 15.1gm)
In 500 ml of a buffer solution containing 0.8 M CH3COOH and 0.6 M CH3COONa , 0.2 M HCl is added. Calculate the pH of Solution before and after adding HCl. (Ka CH3COOH is 1.8x10-5) .
(Ans- i - 4.62 ii- 3.96)
A mixture of 0.2 mole RNH2 and 0.4 mole RNH3Cl is mixed .the volume of Solution prepared is 10 liter (given Kb RNH2 is 10-5) calculate.
(i) pH of resulting solution
(ii) pH of Solution if diluted to 1000 litres
(iii) pH of Solution if 200 ml buffer is mixed with 2 milimoles of H+
(iv) pH of Solution if 200 ml buffer is mixed with 2 milimoles of OH-
(Ans- i- 5.30 , ii- 5.31 , ii- 8.3 , iv -9.0)
Tuesday, October 23, 2018
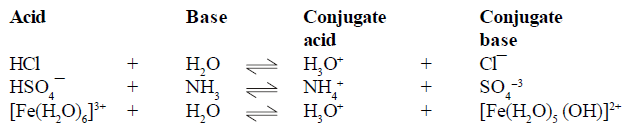
BRONSTED LOWERY ACID-BASE CONCEPT
For example
(1) conjugate pair is acid-base pair differing in single proton (H+)
(2) conjugate acid is written by adding H+ and conjugate is written by removing H+ .
(4) Equilibrium always moves from strong acid to weak acid and strong base to weak base.
MERITES OF BRONSTED CONCEPT:
(1) the role of solvent clearly defined.
(2) the acidic and basic character may be observed in non aqueous medium also .
(3) the acidic ,basic or Amphoteric nature of most of the substance may be defined.
(4) the acid having greater tendency to donate protons are stronger acid and base having greater tendency to accept protons are stronger base .
(5)In conjugate pair ,if one is strong then other must be weak .
The weak acid or base are normally determined by comparing the the stability of different acid or base
DEMERITES OF BRONSTED CONCEPT:
(1) Proton is a nuclear particle hence reaction should not explained in term of proton.
(2) the neutralized process becomes multiples step process.
(3) Most of the Amphoteric solvent become Amphoteric.
AMPHOTERIC SPECIES (Amphiprotic):
The species which have a tendency to donate proton as well as accept proton (H+) such species are known as Amphoteric species.
For example H2O,NH3 HS- ,HPO3- ,HC2OO4- , H2O4 etc
ILLUSTRATIVE EXAMPLES:
(1)The conjugate base of HCO3 is –
(A) H2CO3 (B) CO2 (C) H2O (D) CO3-
(2) The conjugate acid of HSO3- is -
(A) SO32- (B) SO42- (C) H2SO4 (D) H2SO3
(Ans: 1-D 2-D)