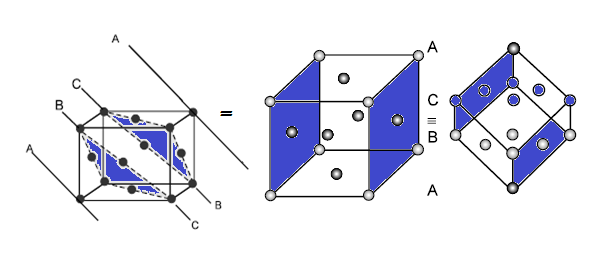
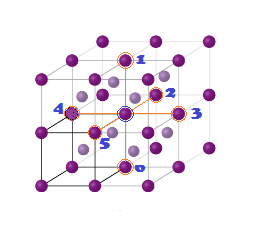
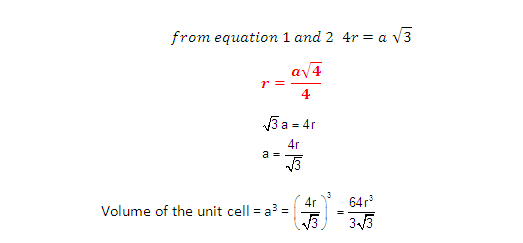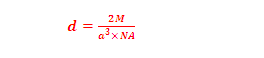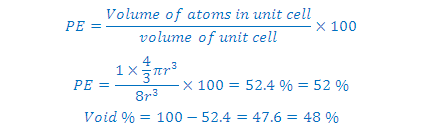CCP or FCC has two lattice point corner as well as face centred:
Suppose
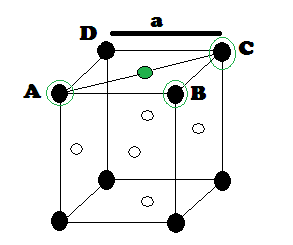
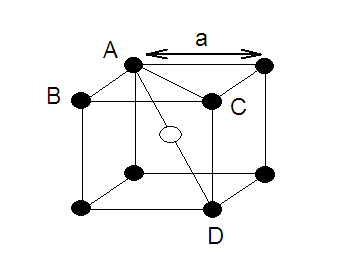
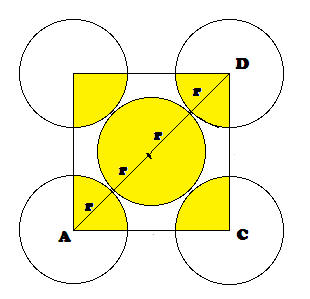
‘r’ be the radius of sphere and ‘a’ be the edge length of the cube As there are
4 sphere in FCC unit cell
(1) Relation between radius (r)
and side (a)
In FCC,
the corner spheres are in touch with the face centred sphere. Therefore, face
diagonal AD is equal to four times the radius of sphere AC = 4r
But from
the right angled triangle ABC:
(2) Effective no. of atoms per unit cell (Z):
(3) Volume of
four spheres (atoms):
(4) Volume of unit cube:
(5) Packing efficiency (PE): Percentage of space occupied by sphere
(6) Percentage Voids: 100- PE=
26 %
(7) Density of FCC(CCP):
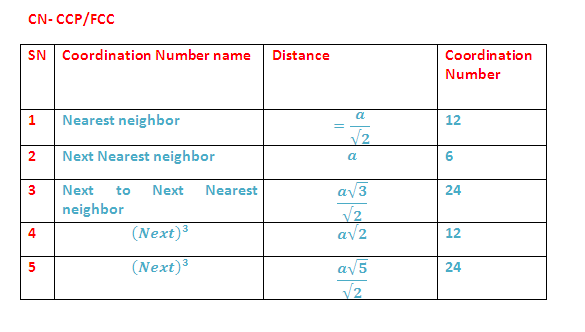
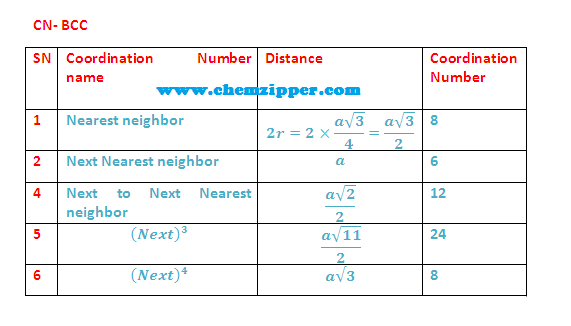
(8) Coordination Numbers:
(9) LOCATION OF VOIDS: FCC/CCP UNIT CELL:
(A) Tetrahedral voids: The
FCC/CCP unit cell has eight tetrahedral voids per unit cell. Just below
every corner of the unit cell, there is one tetrahedral void. As there are eight corners, there are eight tetrahedral voids.
(B) Octahedral voids: In
an FCC/CCP unit cell, there are four octahedral voids. They are present
at all the edge centres and at the body centre. The contribution of the
edge centre is 1/4
Hence, total number of octahedral
voids:
In CCP/FCC:
Rank (Z) = 4,
Number of tetrahedral voids = 8 and
Number
of tetrahedral voids = 2 × Z
Number of tetrahedral voids in close
packing = 2 × eff. no. of spheres.
Hence,
there are two Tetrahedral Voids per sphere in closed packing arrangements.
In CCP/FCC:
Z = 4
Number of octahedral voids = 4
Number of octahedral voids = Z
There
is exactly one OV per sphere in close packing.