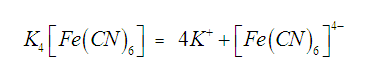The
number of atoms, ions, or molecules that a central atom or
ion holds as its nearest neighbours in a complex or coordination
compound are known as Coordination number or Ligancy.
|
S.N.
|
Metal Cation
|
C.N.
|
Examples
|
|
1
|
Zn2+
|
4
|
[Zn(NH3)4]-
|
|
2
|
Cd2+
|
4
|
[Cd(CN)4]2-
|
|
3
|
Hg2+
|
4
|
[Hg(I)4]2-
|
|
4
|
Hg2+
|
3
|
[Hg(I)3]-
|
|
5
|
Cu1+
|
2
|
[Cu(CN)2]-
|
|
6
|
Cu1+
|
4
|
[Cu(CN)4]3-
|
|
7
|
Cu2+
|
4
|
[Cu(NH3)4]2+
|
|
8
|
Cu2+
|
5
|
[Cu(Cl)5]3-
|
|
9
|
Cu2+
|
6
|
[Cu(H2O)6]++
|
|
10
|
Ag1+
|
2
|
[Ag(CN)2]1-
|
|
11
|
Ag3+
|
4
|
[Ag(F)4]1-
|
|
12
|
Au1+
|
2
|
[Au(CN)2]1-
|
|
13
|
Au3+
|
4
|
[Au(F)4]3-
|
|
14
|
Ni2+
|
4
|
[Ni(Cl)4]2-
|
|
15
|
Ni2+
|
5
|
[Ni(CN)4]3-
|
|
16
|
Ni2+
|
6
|
[Ni(NH3)6]2+
|
|
17
|
Pd2+ /Pt2+
|
4
|
[Pd(Cl)4]2- , [Pt(NH3)4]2+
|
|
18
|
Pd4+ /Pt4+
|
6
|
[Pd(H2O)6]4+
, [Pt(NH3)4]2+
|
|
19
|
Co2+
|
4
|
[Co(SCN)4]2-
|
|
20
|
Co2+
|
6
|
[Co(H2O)6]3+
|
|
21
|
Co3+
|
6
|
[Co(NH3)6]3+
|
|
22
|
Rh3+
|
6
|
[Rh(F)6]3-
|
|
23
|
Ir3+
|
6
|
[Ir(F)6]3-
|
|
24
|
fe2+ /Fe3+
|
6
|
[Fe(CN)6]4- ,
[Fe(CN)6]3-
|
|
25
|
Mn2+
|
4
|
[Mn(Br)4]2-
|
|
26
|
Mn2+
|
5
|
[Mn(Br)5]3-
|
|
27
|
Mn2+
|
6
|
[Mn(NH3)6]2+
|
|
28
|
Sc3+
|
6
|
|
|
29
|
Ti3+
|
6
|
|
|
30
|
V3+
|
6
|
|
|
31
|
Cr2+
|
6
|
|
|
32
|
Cr3+
|
6
|
|