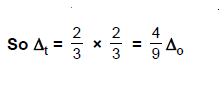Tetahedral
complex (sp3):
In a
tetrahedral field : Consider a cube such that a metal atom or ion is situated
at its centre of symmetry through which the axis of geometry are passing and
joining the face centres of this cube. Therefore, lobes of eg orbitals will be directed
towards the face centres but those of t2g orbitals will be pointing towards
edge centres. Now consider 4 monodentate ligands approaching the metal, the 4 alternate
corners of this cube so as to make a tetrahedron.
Thus it is
clear that t2g
orbitals are nearer to the ligands than the eg orbitals. Hence t2g orbitals
will experience more repulsion than eg orbitals. Therefore, crystal field
splitting will be reversed of octahedral field which can be shown as below.
In
tetrahedral complexes none of the ligand is directly facing any orbital so the
splitting is found to be small in comparison to octahedral complexes. For the
same metal, the same ligands and metal-ligand distances, it can be shown that del.tetra = (4/9) del.oct.
This may attributes to the following two reasons.
(1) There are only four ligands instead of six, so
the ligand field is only two thirds the size; as the ligand field spliting is
also the two thirds the size and
(2) The
direction of the orbitals does not concide with the direction of the ligands.
This reduces the crystal field spliting by roughly further two third.
Consequently,
the orbital splitting energies are not sufficiently large for forcing pairing
and, therefore, low spin configurations are rarely observed.
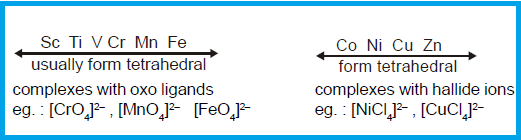
FACTORS FAVOURING TETRAHEDRAL COMPLEXES:
Tetrahedral
complexes are favoured by steric requirements, either simple electrostatic repulsion
of charge ligands or vander wall's repulsions of large one. A valence bond (VB)
point of view ascribed tetrahedral structure to sp3 hybridisation.
Tetrahedral
complexes are thus generally favoured by large ligands like Cl-, B-, I-
and PPh3 and metal ions of
six types;
(1) Those with a noble gas configuration
such as Be2+ (ns0);
(2) Those with pseudo noble gas
configuration (n-1)
d10ns0np0, such as Zn2+, Cu+ and Ga3+, and
(3) Those transition metal ions which do
not strongly favour other structure by virtue of the CFSE, such as Co2+, d7.
(4) Those transition metal which have
lower oxidation state.
(5) Those metals generally with
electronic configuration d0, d5 and d10 prefer
to form such complexes.
(6) It is observed that
OTHER EXAMPLES :
|
SN
|
Complex
|
Nature
|
|
1
|
[Ni(CO)4]
|
Diamagnetic
|
|
2
|
[Ni(Cl)4]2-
|
Paramagnetic with two unpaired
electron
|
|
3
|
[NiCl2(pph3)2]
|
Paramagnetic with two unpaired
electron
|
|
4
|
[MnCl4]2-
|
Paramagnetic with five unpaired
electron
|
|
5
|
[FeCl4]2-
|
Paramagnetic with four unpaired
electron
|
|
6
|
[Cu(py)4]+
|
Diamagnetic
|
|
7
|
Cs2[CuCl4]
|
Paramagnetic with two unpaired
electron (Orange tetrahedral) Sp3
|
|
8
|
NH3[CuCl4]
|
Paramagnetic with two unpaired
electron (Yellow Square Planer) dsp2
|
|
9
|
[Zn(NH3)4]2+
|
(d10) CFSE=0 , Diamagnetic
|
|
10
|
[Zn(CN)4]2-
|
(d10) CFSE=0 , Diamagnetic
|