1. Definition of Back Bond:
(1) Back bonding is a type of weaker π bond that is formed by sideways overlapping of filled orbital with empty orbital present on adjacent bonded atoms in a molecule.
(2) It is also considered an intermolecular Lewis acid-base interaction as it is a π bond.
(3) Back bonding is found to be effective and considerable in the following type of overlapping.
[ (i) 2p-2p (ii) 2p-3p (iii) 2p-3d ]
(4) the extent of overlapping order is
[2p-2p> 2p-3d >2p-3p]
(5) dx2-y2 and dx2 Orbital does not participate in back Bonding.
ILLUSTRATIVE EXAMPLE (1): Which of the following options is/are true about back Bonding?
(A) Sigma-dative bond
(B) π- dative
(C) Intermolecular Lewis acid-base interaction
(D) Intramolecular Lewis acid-base interaction
SOLUTION: Options B and D are responsible for back Bonding and options A and C are responsible for Coordinate Bonding.
2. CONDITIONS FOR BACK BONDING:
(1) Both of the atoms bonded with back Bonding must be present in the 2nd-2nd or 2nd-3rd period.
(2) One of the atoms has lone pair and another has a vacant Orbital and the direction of back Bonding depends upon the vacant Orbital.
(3) The donor atom must have localized donatable electron pair. In general, these are later half-second period P - block elements (F, O, N and C).
(4) The acceptor atom must have low energy empty orbital which generally are np or nd orbitals. Small and similar-sized orbitals favour overlap.
3. EFFECTS OF BACK BONDING:
(1) It always leads to an increase in bond order between the participating atoms.
(2) It always leads to an increase in bond strength between participating atoms.
(3) It always leads to a decrease in bond length between participating atoms.
LEWIS ACID CHARACTER OF BORON HALIDES:

(1) Back bonding extent in boron trihalides decreases from BF3 to BI3 because by increasing the size of the p-orbital of halogen the strength of the back bond decreases. Thus Extent of back Bonding:
[BF3>BCl3>BBr3>BI3]
(2) Lewis acid character of Boron Halides is inversely proportional to the extent of back bonding because on decreasing back bonding tendency to accept lone pair from the base increases thus the order of Lewis acid character is :
[BF3<BCl3<BBr3<BI3]
Thus it is clear that BF3 is the weakest Lewis acid due to stronger 2pπ-2pπ back bonding (stronger partial double bond character) in BF3 (lone pair orbital of fluorine into vacant orbital of boron) and consequently behaves as less electron deficient. The back bonding gradually decreases (From BF3 to BI3) and becomes weakest in BI3. So that BI3 become a strong Lewis acid.
(3) The Nucleophilicity order is inversely proportional to the Lewis acid character thus the Nucleophilicity order is: (reaction with nucleophile/water
[BI3>BBr3>BCl3>BF3]
4. d-ORBITAL RESONANCE:
It is a phenomenon in which electrons of ms and np get delocalized to vacant nd orbital because this availability of vacant d orbital to expect back bond get reduced.
In those molecules species where the d orbital’s resonance exists back, Bonding is decreased.
ILLUSTRATIVE EXAMPLE (2): N(CH3)3 is pyramidal while (SiH3)3N is trigonal planer why?
SOLUTION: N(CH3)3 has sp3 hybridization & pyramidal shape at N, but in (SiH3)3N again there is 2pπ—3dπ back bonding between lone pair orbital of nitrogen into vacant orbital of silicon. Hence trisilyl amines are sp2, planer & is less basic than trimethyl amine.

ILLUSTRATIVE EXAMPLE (3): (1st) N(SiH3)3,(2nd) {(Me)3Si}N---Si(Me)3 and(3rd) HN(SiH3)2
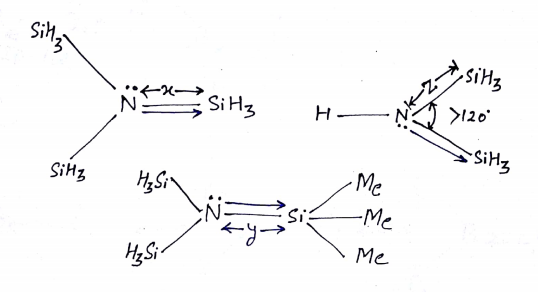
Q (1): Which is greater x or y?
Q (2): Which is greater x or z?
Q (3): Which has a greater extent of back Bonding?
SOLUTION:

Ans: (1) ‘y’ is greater than ‘x’ because of the steric repulsion of the -CH3 group.
Ans: (2)’z’ will be greater because one lone pair going two places.
Ans; (3) the extent of back bonding is 3rd >1st > 2nd
ILLUSTRATIVE EXAMPLE (4): Give the correct order of (B-H) bond length of the following compounds. (1) B(OH)3 (2) B(OMe)3 and(3) B(Me)2OH
SOLUTION:

Extent Back Bonding is 3>1>2 and bond length order is y>x>z
ILLUSTRATIVE EXAMPLE (5): Arrange the silicon halides into decreasing order of Lewis acids Character? SiF3, SiCl3, SiBr3, SiI3
SOLUTION: In the case of silicone halides inductive effect dominate over back bonding hence Lewis acid character is decided by the inductive effect.
Hence order of lewis acid character SiF3 > SiCl3 > SiBr3 > SiI3
ILLUSTRATIVE EXAMPLE (6): Compare the acidic strength of silanol (SiH3OH) and methanol (CH3OH).
SOLUTION: H3C-OH is less acidic than H3Si-OH due to stabilization of negative charge in H3Si-O- ion by 2pπ—3dπ back bonding

ILLUSTRATIVE EXAMPLE (7): Choose the correct statement about the structure of H3BO3 is/are. Statements are as:
(1) Angle OBO =120
(2) Angle HOB>109
(3) Hybridization of atom O close to sp2 and
(4) Molecule is non-planer and non-polar

SOLUTION: Statement (4) is wrong because the molecule is planer and polar
ILLUSTRATIVE EXAMPLE (8): Arrange in increasing order of X-O-X bond angle.
(A) OMe2
(B) O(SiH3)2
(C) O(SiPh3)3
SOLUTION: A>B> C
ILLUSTRATIVE EXAMPLE (9): Arrange increasing order of bond angle (X-O-X) in the following compounds:
(A) OMe2
(B) H2O,
(C) OF2,
(D) OCl2.
SOLUTION: B<A<C<D
ILLUSTRATIVE EXAMPLE (10): Arrange increasing order of bond angle (X-N-X) in the following compounds:
(A) NH3,
(B) NF3,
(C) NCl3
(D) CCl2?
SOLUTION: B<A<C<D
ILLUSTRATIVE EXAMPLE (11): Correct statement about the structure of H3CNCS and H3SiNCS is/are?
(A) CNC bond angle in H3CNCS is >120 and hybridization is closed to sp2
(B) Si-N-C bond angle is 180 in H3CNCS
(C) Both have Back Bonding
(D) Skeleton Si-N-C-S is linear but molecules are non-planer.
SOLUTION: (A, B, D)


Due to back Bonding between nitrogen and silicon atom bond length decreases and shape become linear.
Hence options A, B, and D are correct.
ILLUSTRATIVE EXAMPLE (12): Correct statement about B3O6-3 and B3N3H6?
(A) Both are planer and non planer
(B) Both have aromatic character
(3) Both have a ppi-ppi bonds formed by the pairing of unpaired electrons
(4) Electrophilic reaction occurs at B3N3H6
SOLUTION:

SOLUTION:( A, B, D) In Boraxine ion boron and oxygen atom alternatively combined to form the six-member ring and also each boron atom is linked with extra oxygen atoms. Both boron and oxygen atoms have sp2 hybridization (by Back bonding and all oxygen atom involved in back bonding) and planer structure due to the fact ring become aromatic but due to sp3 hybridisation of the oxygen atom molecule become planer.
In the Borazine molecule, nitrogen is more electro-negative than boron. Nitrogen acquires a partial negative charge and boron acquires a partial positive charge and back bonding takes place between boron and nitrogen.
Even though Borazine and Benzene have the same stricture their chemical properties are different.
(1) Organic benzene is C6H6 while Inorganic benzene is Borazine having chemical formula B3N3H6
(2) Borazine is more reactive than Benzene with respect to addition reactions.
(3) Aromaticity of borazine is less than benzene hence it is less reactive toward Electrophilic substitution reactions
Hence options A B and D are correct.
5. BACK BONDING: CONCLUSIONS:
(1) Due to back Bonding, bond length always decreases.
(2) If the empty atomic orbital of the central atom of the molecule participates in back bonding then its hybridization does not change and its Lewis acid Character decreases.
(3) If the filled orbital of the central atom of a molecule participates in back Bonding then its hybridization may change and its Lewis basic Character may also change for example N(SiH3)3, however in some molecules, hybridization may not change.
(4)Due to back Bonding, bond angle either increases or remains the same but never decreases.
(5)In most molecules steric factor enhance (increase) the extent of back Bonding, for example, N(SiH3)3, OCl2, NCl3, O(SiH3)2 (disilyl ether) however in some cases steric Factors decreases extent of back Bonding for example O3BMe3, NSi(Me3)(N3).
(6) When the skeleton is planer then the steric Factor decreases the extent of back bonding.
(7) In the 2p-2p type of back Bonding, back Bonding dominates over the inductive effect while in 2p-3d and 2p-3p inductive effect dominates over back Bonding.
(8) Me3NO has a greater dipole moment than Me3PO as there is 2pπ—3dπ back donation from Oxygen into vacant d-orbitals of phosphorus (just like in CO).
(9) Me3C-OH is less acidic than Me3Si-OH due to stabilization of negative charge in Me3Si-O- ion by 2pπ—3dπ back bonding.
(10) Me2O forms an adduct with BF3 but (SiH3)2O does not react with BF3 due to the weakening of the basic character of Disilyl ether by back bonding.
(11) BH3 does not exist (it exists only as a dimer or higher boranes) but BX3 exist, (X=halogen). It can be attributed to the absence of the possibility of back bonding in BH3.
(12) BF3 is only partially hydrolysed into [BF3(OH)]- whereas BCl3 & BBr3 are completely hydrolysed into B(OH)3 or H3BO3 and HCl/HBr
(13) B-F bond length increases when BF3(130 pm) reacts with F- to form (BF4)- [143 pm]. It is due to the absence of Back-bonding in (BF4)- hence B-F bond has a completely single bond character.
(14) Si-O and P-O bonds are much stronger than expected to partial double character owing to the possibility of back-bonding.
(15) Bond angle of NF3(102 degrees) is lesser than in NH3 (107) as per VSEPR theory which suggests that in the case of less electronegative terminal atoms like H, Bond pairs would be closer to the more electronegative central atom, N and hence bonds open up due to repulsion between bond pairs electron density in the vicinity. But the bond angle of PF3 (100) is greater than PH3, it is due to the possibility of back bonding in PF3 between lone pair of fluorine and vacant d-orbital of phosphorous (2pπ—3dπ) henceforth P-F bond acquires partial double character and we know well that multiple bonds cause more repulsion so the bond angle is greater.
(16) SiCl4 has an abnormally low boiling point than CCl4,
(17) Due to the possibility of Back-bonding with metal (similar to carbonyl complexes), Ph3P or R3P or PF3 behave as strong ligands in complexes.
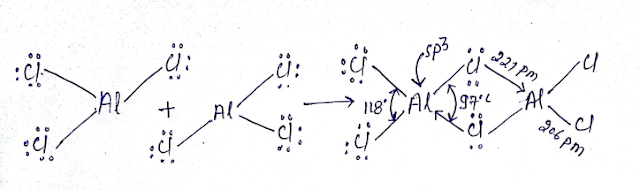
NOTE- 3pπ—3pπ Back bonding in AlCl3 is not as effective hence it easily forms dimer in vapor phase or non-polar solvent.
6. BACK BONDING IN METAL CARBONYL:
(1) The carbon atom in carbon monoxide has a single pair of electrons that can be used to form a sigma bond with a metal. Because carbon monoxide has a low-lying orbital, it can accept electrons back from the metal, strengthening the bond between the metal and the carbon monoxide ligand even more. Back bonding refers to the process of "accepting electrons back from the metal." It is critical to understand that because metal is more negatively charged, M-C back-bonding is stronger and C-O bonding is weaker than in CO.
(2)Back bonding is mostly observed in CO ligands which is a sigma donor as well as a pi- acceptor. [The typical example given for synergy in chemistry is the synergic bonding seen in transition metal carbonyl complexes. CO has much less dipole moment (0.11D) than expected due to back-donation from lone pair orbital of Oxygen into vacant orbital of carbon. (Similar behaviour from nitric acid, NO)
(3)Back bonding is also common in the organometallic chemistry of transition metals which have multi-atomic ligands such as carbon monoxide, ethylene or the nitrosonium cation e.g, Ni(CO)4 and Zeise’s salt, K[PtCl3(C2H4)]