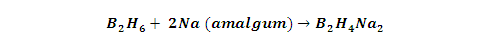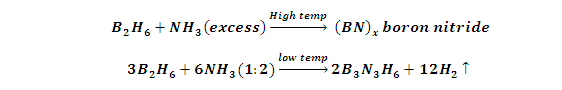Search This Blog
Thursday, February 28, 2019
MOLE-MOLE ANALYSIS:
Monday, February 25, 2019
PERCENTAGE(%) YIELD OF CHEMICAL REACTION:
Initially 2.5 moles of Fe(NO3)3 and 3.6 moles of Na2CO3 are taken. If 6.3 moles of Na2CO3 is obtained the % yield of given reaction is ?
Sunday, February 24, 2019
PERCENTAGE(%) PURITY OF GIVEN SAMPLES:
Wednesday, February 20, 2019
HEISENBERG'S UNCERTAINTY PRINCIPLE:
ILLUSTRATIVE EXAMPLE (4): If uncertainty in position and momentum of electron are equal then prove that uncertainty in velocity is ...
ILLUSTRATIVE EXAMPLE (5):If uncertainty in momentum of an electron are three times of uncertainty in position then uncertainty in velocity of electron would be
ILLUSTRATIVE EXAMPLE (6): What is the uncertainty of Photon in position of wave length 500 A .If wave length is known to an accuracy of 1pm.
ILLUSTRATIVE EXAMPLE (7): An electron is accelerated by (V) volt and following graph is obtained calculate the (V) voltage?
ILLUSTRATIVE EXAMPLE (8): A electron having velocity 2×10+6 m/s has uncertainty in kinetic energy is 6.62/π×10-34 j, than calculate the uncertainty in position of electron in Anstrom .
ILLUSTRATIVE EXAMPLE (9): Two particles A and B are in motion .if the wave length associated with particle A is 5×10-8 m. Calculate the wave length associated with particle B if momentum is Half of A?
ILLUSTRATIVE EXAMPLE (10): If uncertainty in position of an moving electron is equal to its de Broglie wave length, then its velocity will be completely uncertain. Explain?
ILLUSTRATIVE EXAMPLE (11): If the de Broglie wave length of a particle of mass (m) is 100 times of its Velocity. Then its value in term of its mass (m) and plank constant (h) is?
Saturday, February 2, 2019
DIBORANE-HYDRIDE OF BORONE-(B2H6):
INTRODUCTION:
Boranes are hydride of Boron and diborane is famous
borane. It is gas and is highly inflammable in air and poisonous Diborane is
used for preparing substances such as high energy fuel and propellants.
CHEMICAL PROPERTIES OF DIBORANE:
The Boranes unddergo
different type of chemical reactions like oxidation, pyrolysis, Nucleophilic
and electrophic and reactions with bases
such as OH- and NH3.
(1) Reaction
with Na: Diborane reacts with sodium amalgum to form an
addition product B2H6Na2.
(3) Thermal Stability: B2H6 stable
only at low temperature when heated 100 to 250 degree it changes into higher
hydrides and On heating to 700ºC diborane dissociates.
Note: - Formation of B2H2Cl4 shows that the 2H left in B2H2Cl4 are responsible for dimmer formation (Bridge bond). Diborane has only four replaceable hydrogen and with their replacement, the dimeric structure continuous to be as such. Remaining two hydrogen when they get displaced, the dimeric structure break indicating that these two hydrogen are act as bridging hydrogen.
(9) Reaction with Ammonia (NH3):
(1) Diborane react with excess NH3 at temperature to form (inorganic graphite) Boron nitride (BN) x.while when diborane and NH3 react in 1:2 ratios at low temperature give Inorganic Benzene (Borazole).
(2) Diborane
is electron-deficient molecule and hence it reacts with several molecules
having lone pair(s) of electron like CO, ether, amines, to
form complex compounds.
(3) B2H6 give symmetrical
cleavage with respect to only large
size and weak amines CO, H¯, N(CH3)3 ,
THF, PH3, PF3 , OEt3 OMe2 ,Pyridine,
Thiophene ,SMe2, Set3 etc.
(4) In
the presence of small and strong base B2H6 undergo unsymmetrical
cleavage like NH3, H2N
(CH3), HN (CH3)2 etc
(10)
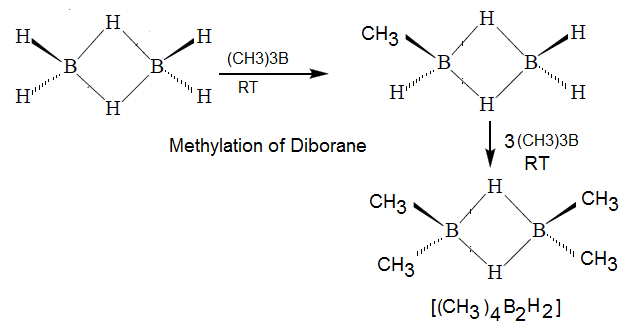
Methylation of Doborane:
(1) In diborane two boron atoms and four terminal hydrogen atoms lie in one
plane. While two bridge hydogen atoms (encircled ) lie smmetrically above and
below the plane.
(2) Total valence electrons in B2H6 is 12(6 from boron
3x2) and 6 from six hydrogen atoms) and there are two B-H-B (3C-2e) bridge
bonds and four B-H (2C-2e) terminal bonds.
(3) Bond energy of B-H-B bond is 441 kjpermole
which greater than bond energy B-H bond (341Kjpermole)
hence methylation of diborane no more than four hydrogen.
(4) In above
reaction it is clearly reveal that none of the bridge hydrogen in B2H6
has been replaced by –CH3 .ie
in this reaction both the bridge bond remaine undissociate.
Structure of "Inorganic Benzene" /Borazole/Borazine:
PHYSICAL PROPERTIES DIBORANE:
(1) Diborane is a colourless gas with a foul smell and is extremely
toxic.
(2) Melting point = -164.85oc and
Boiling point = -92.59oc
(3) It is an extremely reactive inflammable gas which burns in air with a green flame
Structure of Boron nitride (Inorganic Graphite):
Diborane react with excess NH3 at
temperature to form boron
nitride (BN) x.while when diborane and NH3 react
in 1:2 ratios at low temperature give Borazole.
Boron
Nitrides exist two forms just like allotropic forms of carbon (Graphite and Diamond) and both have formula (BN)x.
(i) Boron Nitride (Inorganic Graphite):
(1) Boron nitride is a hexagonal 2D planar giant covalent network , slippery and a white solid with a layered structure like graphite. Doe to similar structure with graphite it know as “Inorganic graphite” and due to white colour it is also called “white graphite”
(2) The thermodynamically stable phase of boron nitride, BN, consists of planar sheets of atoms like those in graphite The planar sheets of alternating B and N atoms consist of edge shared hexagons and, as in graphite, the B-N distance within the sheet (145 pm) is much shorter than the distance between the sheets (333 pm,). The difference between the structures of graphite and boron nitride, however, lies in the register of the atoms of neighboring sheets:
(3) The B-N-B or N-B-N bond angle is 120oc . It may be expected for perfect hexagonal ring bond network just like graphite. And boron and nitrogen atoms are sp2 hybridized.
(4) Boron nitride (Inorganic
graphite ) is a very good insulator (thermal and electrical) and chemically
very inert , chemically posses great stability due to the very strong B-N bonding in the 2D layers structure. It melts under
pressure at 3000oc so it is great thermal stability.
(5) In
(BN)x the hexagonal rings are stacked directly over each other,
with B and N atoms alternating in successive layers; in graphite, the hexagons
are staggered. Molecular orbital calculations suggest that the stacking
(6) In
(BN)x stems from a partial positive charge on B and a partial
negative charge on N. This charge distribution is consistent with the
electronegativity difference of the two elements.
(7)In Boron nitride(BN)x the Vander Waals forces holding the sheet in line with each
other are stronger, so boron nitride is not as good a good lubricant as
graphite. However , the use of boron nitride as a lubricant is noted as
high temperature due to its chemical stability.
(8) As with impure graphite,
layered boron nitride is a slippery material that is used as a lubricant.
Unlike graphite, however, it is a colorless electrical insulator, as there is a
large energy gap between the filled and vacant π bands
Uses:
(1) Boron nitride ceramics us in high temperature (range 2700-3000oc) equipment due to excellent thermal stability, thermal shock stability and chemical stability.
(2) Boron nitride based ceramics are stable in air at 1000oc while carbon-graphite based materials ignited at that temperature.
(3) Hexagonal boron nitride can be made in single layers and can also be formed into nanotubes. And that nanotubes are used for wire sieving and a catalyst support.
(4) Hexagonal boron nitride can be
incorporated in ceramics, alloys, resins, plastics, rubbers to give them
self-lubricating properties. And plastics based hexagonal boron nitride decrease thermal expansion, increased thermal
conductivity, increased electrical insulation.