SOLUBILITY OF GASES AND HENRY'S LAW:
Solubility:
Solubility of a substance is its maximum amount that can be dissolved in a specified amount of solvent at a specific temperature. It depends upon the nature of solute and solvent as well as temperature and pressure.
Unit: Unit of Solubility is gm/litre or Mole/Litre
(A) Solubility of Solid in a Liquid:
Every solid does not dissolve in a given liquid. While sodium chloride and sugar dissolve readily in water, naphthalene and anthracene do not. On the other hand, naphthalene and anthracene dissolve readily in benzene but sodium chloride and sugar do not.
Every solid does not dissolve in a given liquid. While sodium chloride and sugar dissolve readily in water, naphthalene and anthracene do not. On the other hand, naphthalene and anthracene dissolve readily in benzene but sodium chloride and sugar do not.
(1) It is clear observed that polar solutes dissolve in polar solvents and non polar solutes in non-polar solvents.
(2) In general, a solute dissolves in a solvent if the intermolecular interactions are similar in the two or we may say like dissolves like.
(3) Dissolution: When a solid solute is added to the solvent, some solute dissolves and its concentration increases in solution. This process is known asdissolution.
(4) Crystallisation: Some solute particles in solution collide with the solid solute particles and get separated out of solution. This process is known as crystallisation.
"A stage is reached when the two processes (dissolution and crystallisation) occur at the same rate. Under such conditions, number of solute particles going into solution will be equal to the solute particles separating out and a state of dynamic equilibrium is reached.
At this stage the concentration of solute in solution will remain constant under the given conditions, i.e., temperature and pressure. Similar process is followed when gases are dissolved in liquid solvents.
(5) Saturated solution: Such a solution in which no more solute can be dissolved at the same temperature and pressure is called a saturated solution.
(6) Unsaturated solution: The Solution in which more solute can be dissolved at the same temperature.
(7) The solution which is in dynamic equilibrium with undissolved solute is the saturated solution and contains the maximum amount of solute dissolved in a given amount of solvent. Thus, the concentration of solute in such a solution is its solubility.

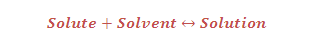
No comments:
Post a Comment