We know that acidic strength of the acid also depends upon stability of conjugate bases, so for relative strength of acid, we need to check the relative stabilities of their conjugate bases.
CF3-, CCl3-, CBr3- CI3-We are expecting the acidic strength haloform acids as
CHF3, CHCl3, CHBr3, CHI3 in decreasing order. Because Fluorine is most electronegative atom so it would be stabilize CF
3- more, as electronegativity decreases from F to I the stability of conjugate -ve ion would be but that is not correct the actual order is
CHCl3 > CHF3 > CHBr3 > CHI3.This is because there is effective
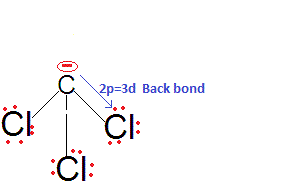
back bonding in CCl3-and hence the negative charge partially gets stabilised by back donation to the vacant 3d orbitals of Cl. Thus, CHCl
3 is a stronger acid than CHF
3 and also among them due to
2pπ-3dπ back bonding.The acidic strengths of the other three haloforms can be compared the inductive effects of their anions. F is very electronegative and hence stabilises the negative charge on the C atom. So, CHF3 is a better acid than CHBr3, and the least acidic is CHI3.
The overall acidic strength order is:
CHCl3 > CHF3 > CHBr3 > CHI3.