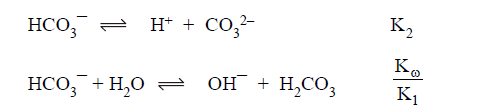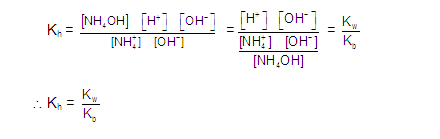(1) Effect of temperature on Solubility
(2) Effect of common ions on Solubility
(3) Effect of simultaneous Solubility
(4) Effect of solvent on Solubility
(5) Effect of pH on Solubility
(i) Effect of pH on Solubility of metal hydroxide
(ii) Effect of pH on Solubility of salt of weak acid
(iii) Effect of pH on Solubility of salt of strong acid
(6) Effect of buffer solution on Solubility
(7) Effect of complex formation on Solubility
In general most cases Solubility increases on temperature . however we must follow two cases .
(1) In endothermic reaction Solubility Increases on increasing temperature.
(2) In exothermic reaction Solubility decrease on increasing temperature .
(1) Solubility of a salt decrease in the presence of common ion.
(2) Higher the concentration of common ion smaller Solubility.
ILLUSTRATIVE EXAMPLE (1): Calculate the Solubility of AgCl in .. ( Kal AgCl is 10-10)
(1) In pure water
(2) In 0.1M NaCl
(3) In 0.01 M NaCl
(4) In 10-5 M NaCl
(5) In 0.05 M HgCl2
(6) In 0.01 M AgCl
(7) In 0.1M KNO3
SOLUTION :
(a) AgCl Ksp = 2×10-10
(b) BaSO4 Ksp = 4×10-8
(C) CaF2. Ksp = 1.08×10-10
(D) Hg2I2. Ksp = 9×10-17
SOLUTION:
ILLUSTRATIVE EXAMPLE (1):
Calculate simultaneous Solubility of AgSCN and AgBr in water . ( Ksp AgSCN=10-12 Ksp AgBr =5×10-13)
CaCO3 and BaSO4 have Solubility products value 1.0×10-8 and 5.0× 10-9 respectively, if water is shaken up with both solids till equilibrium is reached calculate the concentration of CO3-2 ion is ?
The Ksp value of CaCO3 and CaC2O4 in water are 4.7 × 10-9 and 1.3 ×10-9, respectively , at 25°c .If mixture of two is washed with water , what is Ca2+ ion concentration in water ?
(Ans- 7.707×10-5 )
ILLUSTRATIVE EXAMPLE (4):
Solubility of solute in solvent purely depends on nature of both solute and solvent , a polar solute dissolved in polar solvent and non polar solute dissolved in non polar solvent . A polar solute has low solubility or insoluble in a non polar solvent . For this reason if you want to decrease the Solubility of an inorganic salt (polar salt ) in water you mixed the water with an organic solvent (non polar )
We know that:
IP= Ionic product and Ksp= Solubility product
CASE(1): If IP < Ksp then Solution is unsaturated
CASE(1):If IP > Ksp then Solution is Oversaturated or ppt formation occurs.
CASE(1): If IP =Ksp then Solution is saturated is No more solute dissolved.
A 200 ml of 1.3×10-3 M AgNO3 is mixed with 100 ml of 4.5×10-5 M Na2S Solution will precipitatation occurs ?
(Ksp = 1.6×10-19) .
50ml of 6.9×10-3M CaCl2 mixed with 30 ml of 0.04 M NaF2. Will precipitatation of CaF2 occurs ?
( Ksp for CaF2= 4.0×10-11)
ILLUSTRATIVE EXAMPLE (3):
How much solid Pb(NO3)2 must be added to 1.0 L of 0.0010 M NaSO4 Solution for precipitatation of PbSO4 (Ksp=1.6×10-8) to form.
(assume no change in volume when the solid is added).
PbCl2 has Ksp=1.6×10-5 , If equal volume of 0.030 M PB(NO3)3 and 0.030M KCl are mixed , will precipitatation occurs ?
ILLUSTRATIVE EXAMPLE (5):
Many weak soluble ionic compound have Solubility which depends upon the pH of the Solution for example metal hydroxide and salt of weak acids.
ILLUSTRATIVE EXAMPLE (1): Zince hydroxide ( Zn(OH)2 ) has Ksp 4.5×10-17 in pure water calculate it's molar Solubility and pH of resulting solution .
(Ans- S=2.2×10-6 M and pH = 8.6434 )
ILLUSTRATIVE EXAMPLE (2): At what pH the Zince hydroxide will start precipitate (pHs) and at what pH precipitation is completed (pHc) from the Solution containing 0.1M Zn+2 ? ( Given Ksp of Zn(OH)2 is 4.5×10-17)
(Ans- pHs = 6.33 and pHc = 8.33 )
(Given Ksp of Ca (OH)2 is 5.5×10-6 and Ksp of Mg(OH)2 is 5.0×10-12)
For salt of weak acids (eg Sulphides , Carbonate , Oxalates , and Phosphates ) the smaller the value of Ksp the lower the pH at which the salt precipitate (pH ---Ksp) exactly same as metal hydroxide .
It is noted that the salt having smaller Ksp , precipitate in more acidic medium and other hand the salts having Higher Ksp , precipitate in less acidic medium.
For example the precipitatation of Ca +2 at low pH , CO3-2 will be turned to HCO3-1 Or may be to H2CO3 , below at pH=< 8( see the Diagram) while at pH >=13 all the carbonic acid species are present as CO3-2 , therefore CaCO3 will precipitate in basic medium and will dissolve in acidic medium .
Which one will precipitate in more acidic medium CaCO3 (Ksp=4.8×10-9) or MgSO4 (Ksp=1.0×10-5) ?
A solution containing 0.1 M Ti+ and 0.05 M Cd+2 . Is it possible to separate these two ions by precipitatating one of as Sulphides ?
( Ksp(CdS)=2.0×10-28 , Ksp(TiS)=2.0×10-22)
Note that the pH has no effect on Solubility of strong acids salts eg Cl- Br- and SO4-2 etc because the concentration of of these conjugated base in the same either in acidic or basic medium . However metal ions can be separated by these anions according to their Ksp value as the hydroxide or salts of weak acids.
Ca(OH)2 has Ksp = 7.9 ×10-6 , what is the pH of Solution made by equilibrating solid Ca(OH)2 with water ?
ILLUSTRATIVE EXAMPLE (2):
Cu(OH)2 has Ksp =1.6×10-19 calculate the
(1) What is the pH of a saturated solution of Cu(OH)2 ?
(2) What is the maximum Cu+2 concentration possible in a Neutral Solution ? (pH=7)
(3) What is maximum pH of Solution in which concentration of Cu+2 is 0.50 M.
Whenever a salt dissolved in a buffer solution it's pH remain same .
ILLUSTRATIVE EXAMPLE (1):
The Solubility of Pb(OH)2 in water =6.70×10-6. calculate it's Solubility in a buffer solution of pH=8 . [ IIT-1999]
ILLUSTRATIVE EXAMPLE (2):
Calculate Solubility of AgCN in a buffer of pH=3 . ( Given Ksp=1.2×10-15 and Ka HCN is 4.8×10-10 ).
ILLUSTRATIVE EXAMPLE (3):
Calculate Solubility of AgCN in a buffer of pH=3 . ( Given Ksp=1.2×10-16 and Ka HCN is 4.8×10-10 ).
ILLUSTRATIVE EXAMPLE (4):
Calculate the molarity Solubility of Cu(OH)2 [Ksp=2.2×10-20] in
(1) Distilled water
(2) pH =13.0 NaOH (aq)
(3) pH= 4.0 buffer
ILLUSTRATIVE EXAMPLE (5):
ILLUSTRATIVE EXAMPLE (4):
Calculate Solubility of AgCN in a buffer of pH=3 . ( Given Ksp=8×10-10 and Ka HCN is 9×10-10 ).
The Solubility of many salt can be increased by addition of a species that can form complex ion with one of the ions (usually the cation ) formed when poorly soluble salt dissolved.
ILLUSTRATIVE EXAMPLE (1):
Find Solubility (s) of AgCl in 'C'M NH3 (aq) (given , Ksp of AgCl and Kf [Ag(NH3)2]+.
SOLUTION:
[Kf =complex formation constant=K solubility constant=1/K(insolubility)=1/K(dissociation)]
Calculate Solubility of AgCl(s) in 2.7 M NH3(aq) solution . ( Ksp AgCl=10-10 and Kf[Ag(NH3)2]=1.6×10+7).
SOLUTION:
ILLUSTRATIVE EXAMPLE (3):
Calculate the Solubility of AgCN in 0.01M KCN Solution assuming
(1) No Complex formation
(2) Complex formation
(KspAgCN=8×10-8 and Kf[Ag(CAN)2]=4×10+7)
Find the Solubility of MX(s) ( Ksp=1.6×10-10) in 0.05M NaCN(aq).
(1/K dissociation [M(CN2)]=2.5×10-8 )
What is Molar solubility of AgBr in 0.1M NaS2O3 ? ( Given Ksp AgBr = 5×10-13 and Kf [Ag(S2O3)2]-3 =5×10+13)
ILLUSTRATIVE EXAMPLE (7):
Calculate the Molar Solubility of AgBr in 1.0 M NH3 at 25° ( Ksp AgBr =5×10-13 and Kf [Ag(NH3)2]+ =1.7×10+7)
ILLUSTRATIVE EXAMPLE (6):