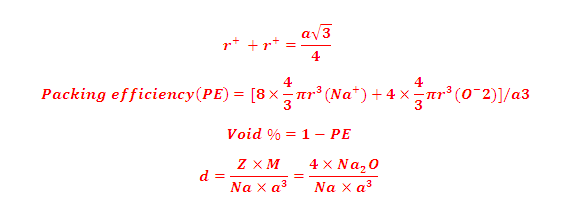The
caesium chloride crystal is composed of equal number of caesium (Cs+)
and Chloride Cl- ions. The radii of two ions (Cs+ = 169 pm
and Cl- = 181 pm) led to radius ratio of Cs+ to Cl- as 0.93 which suggest
a body centred cubic structure having a cubic hole
The salient features of this structure are as follows:
(1) The chloride ion form the simple cubic arrangement and the caesium
ions occupy the cubic interstitial holes. In other words Cl- ions
are at the corners of a cube whereas Cs+ ion is at the centre of the
cube or vice versa
(2) Each Cs+ ion is surrounded by 8 Cl- ions and
each Cl- ion in surrounded by 8 Cs+ ions. Thus the Co –
ordination number of each ion is eight.
(3) For exact fitting of Cs+ ions in the cubic voids the
ratio r Cs+/rCl- should be equal to 0.732. However, actually
the ratio is slightly larger (0.93). Therefore packing of Cl- ions
slightly open up to accommodate Cs+ ions.
(4) The unit cell of caesium chloride has one Cs+ ion and
one Cl- ion as calculated below
No. of Cl- ion = 8(at
corners) ´1/8
= 1
No. of Cs+ ion = 1(at
body centre)´1=1
Thus, number of CsCl units per unit cell is 1
(5) Relation between radius of cation and anion and edge length of the
cube,
Effect of temperature on crystal structure:
Increase of temperature decreases the coordination of number, e.g. upon heating to
760 K, the CsCl type crystal structure having coordination 8:8 changed to NaCl type crystal structures having coordination 6:6.
Effect of pressure on crystal structure:
Increase of pressure increases the Co – ordination number during crystallization e.g. by applying pressure, the NaCl type crystal structure having 6:6 coordination number changes to CsCl type crystal having coordination number 8:8
Other common examples of this type of structure are CsBr, CsI, TlCl, TlBr, TlI and TlCN
Higher coordination number in CsCl(8:8) suggest that the caesium chloride lattice is more stable than the sodium chloride lattice in which Co – ordination number is 6:6. Actually the caesium chloride lattice is found to be 1% more stable than the sodium chloride lattice. Then the question arises why NaCl and other similar compounds do not have CsCl type
lattice – This is due to their smaller radius ratio. Any attempt to pack 8 anions around the relatively small cation (Li+, Na+, K+, Rb+) will produce a state in which negative ions will touch each other, sooner they approach a positive ion. This causes unstability to the lattice.