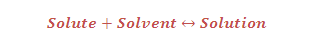VAPOUR PRESSURE:
(1) If a sample of water in its liquid phase is placed in an empty container, some of it will vaporize to form gaseous of water. This change is called evaporation.
(2) The pressure exerted by the vapour (molecules in the vapour phase) over the surface of the liquid at the equilibrium at given temperature is called the vapour pressure of the liquid.
OR
(3) It is the pressure exerted by the vapour when vapours are equilibrium with the liquid.
(4) The pressure exerted by vapours is called unsaturated vapour pressure or partial vapour at non equilibrium condition.
Factors affecting vapour pressure:
(A) Temperature:.
(1) The temperature at which the vapour pressure of the liquid becomes equal to the atmospheric pressure is called its boiling point.
(2) Vapour pressure is directly proportional to theTemperature so that on increasing temperature the rate of evaporation increases and rate of condensation decreases and hence vapour pressure increases.
(3) The dependence of vapour pressure and temperature is given by CLASIUS CLAPERON equation.
(4) Vapour pressure of a particular liquid system is only the function of temperature only. It is independent from all other factors like surface area, amount of liquid, available space etc.
(A) Nature of liquid:
Vapour pressure of liquid =1/the strength of intermolecular forces acting between molecules
For example: CCl4 has higher vapour pressure because of the weak intermolecular forces acting between its molecules than water which has stronger intermolecular forces acting between water molecules of volatile liquid has lower boiling point than a non-volatile liquid.
Note:
(1) Relative lowering of vapour pressure of a solvent is a colligative property equal to the vapour pressure of the pure solvent minus the vapour pressure of the solution.
(2) For example: water at 20°C has a vapour pressure of 17.54 mmHg. Ethylene glycol is a liquid whose vapour pressure at 20°C is relatively low, an aqueous solution containing 0.010 mole fraction of ethylene glycol has a vapour pressure of 17.36 mmHg. Thus the vapour pressure lowering, DP = 17.54 mmHg ¾ 17.36 mmHg = 0.18 mmHg.