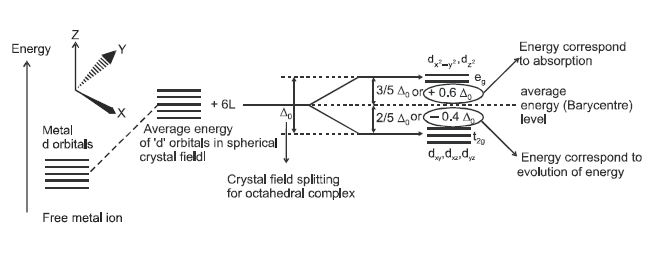
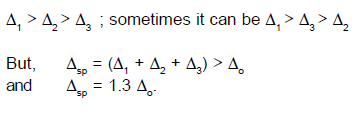For
convenience, let us assume that the six ligands are positioned symmetrically
along the Cartesian axes, with the metal atom at the origin. As the ligands
approach, first there is an increase in the energy of d orbitals to that of the
free ion just as would be the case in a spherical filed. Next, the orbitals
lying along the axes (dz2and dx2-y2 d)
get repelled more strongly than dxy, dyz and dxz
orbitals, which have lobes directed between the axes. The dxy , dyz , dxz
orbitals are lowered in energy relative to the average energy in the spherical
crystalfiled.
Thus, the degenerate set of d orbitals get split into two sets: the lower
energy orbitals set, t2g and the higher energy, eg set.
The energy separation is denoted by del.oct (the subscript o is for octahedral.
Crystal field stabilisation energy (CFSE):
The difference in energy of eg and t2g
Orbitals are called crystal field stabilisation energy
(CFSE):
Where
m
and n = are
number of electrons in t2g
and eg
orbitals respectively and del.oct
is crystalfield splitting energy in octahedral Complexes.
l =
represents the number of extra electron pair formed because of the ligands in
comparison to normal degenerate configuration.
P= (Pairing
energy) the energy required for electron pairing in a single orbital.
The actual configuration of complex adopted is decided by the relative values
of delta and P
Case (1):
If del.oct
is less than P
We have so called weak field or high spin situation, the fourth electron
entered one of the eg orbitals giving configuration (t2g3
and eg1) If now 5th
electron is added to a weak field the configuration become (t2g3
and eg2).
Case (2):
If del.oct
is more than P , we have the strong field , low spin situation and
pairing will occur in the t2g
level with eg
level remaining unoccupied in entities of d1 and d6 ions .