Search This Blog
Thursday, January 23, 2020
Arrange in correct order of basic Character of aniline, pyrrol, pyridine and piperidine?
We know that aliphatic amines are more basic than aromatic amines due to non delocalized of lone pair of nitrogen atom hence piperidine (option IV) is more basic than others
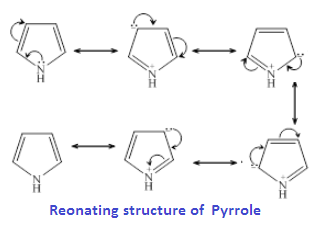
Pyridine is more basic than aniline and pyrrol because is lone pair of nitrogen is does not involved in pi cloud formation that means it is localised while pryrrol is less basic than aniline because is lone pair involved in pi cloud formation of pyrrol ring
Hence correct basic order is (iV) >(iii)>(I)>(ii)
Related Questions:
Wednesday, January 22, 2020
What is correct basicity order of pyridine, pyridazine, pyrimidine and pyrazine ?
Pyridine is more basic as compared to pyridazine,
pyrimidine and pyrazine because pyridazine, pyrimidine and pyrazine containing
two nitrogen atoms which are exerted inductive effect to each other so
availability of lone pair for donation is less.
But
pyridazineis more basic as expected, it may be due to
electrostatic repulsion of the lone pairs of the vicinal nitrogens. This
repulsion destabilizes the non-protonated structure and therefore makes
protonation easier.
Related Questions:
(1) Give an
explanation for the fact that Guanidine NH=C(CH3)2 is a stronger base than most
of amines?
(2) Arrange in
correct order of basic Character of aniline, pyrrol, pyridine and piperidine?
(3) What is correct
basicity order of pyridine, pyridazine, pyrimidine and pyrazine ?
(4) Why pyridine is
more basic than Pyrrole?
(5) Why pyrimidine
is less basic than pyridine?
(6) Imidazole is
more basic than pyridine? Why?
(7) Biological
Important of Imidazole and structure:
(8) Pyridine is
almost 1 million times less basic than piperidine? Why?
(9) Cyclohexylamine
amine is the stronger base than Aniline? Why?
(10) Tetrahydroquinoline
amine is the stronger base than Tetrahydroisoquinoline? Why?
Why pyridine is more basic than Pyrrole?
While in case pyridine
already has a stable conjugated system of three double bonds in an aromatic ring,
like benzene. Hence the lone pair electrons on the N atom in pyridine is
localized and more available for donation
and easily donated to a H+ ions. Therefore, pyridine is a stronger base than Pyrrole.
Related Questions:
Why pyrimidine is less basic than pyridine?
Pyrimidine is less basic than pyridine because (–I
effect) negative inductive
effect of nitrogen atom to another nitrogen atom that cause decreases electron
density of each other. This makes it less willing to donate its lone pair. This
is not count that number of lone pairs to explain basicity.
Related Questions:
Subscribe to:
Comments (Atom)