(1) Introduction:
(1) INTRODUCTION:
The
aldehydes having absence of α-hydrogens when treated with concentrate strong
base undergoes to disproportionate reaction and furnish an alcohol and a carboxylic acid
is called Cannizzaro reaction. In this reaction one molecule of aldehyde is reduced to the corresponding alcohol, while a second one is oxidized to the carboxylic
acid.
(2) MECHANISM OF CANNIZARO REACTION:
Step – (1):
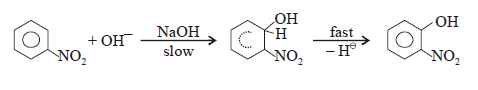
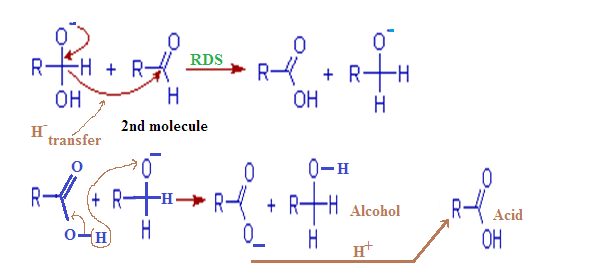
The cannizzaro
reaction is initiated by the nucleophilic attack of a hydroxide ion to the
carbonyl carbon of an aldehyde molecule by giving a hydrate anion. This hydrate
anion can be deprotonated to give an anion in a strongly alkaline medium.
Step – (2):
In this second
step, the hydroxide behaves as a base. Now a hydride ion, H- is
transferred either from the mono anionic
species onto the carbonyl carbon of another
aldehyde molecule. The strong electron donating effect of O-
groups facilitates the hydride transfer and drives the reaction further. This is the
rate determining step of the reaction.
Thus
one molecule is oxidized to carboxylic acid and the other one is reduced to an
alcohol.
(3) CONDITION FOR
CANNIZARO REACTION:
(1) Primary
condition of cannizaro reaction is absence of alpha hydrogen in aldehyde but
(CH3)2CH-CHO gives cannizaro reaction although it has one alpha
hydrogen.
(2) CCl3-CHO
does not give cannizaro reaction while it has no alpha hydrogen it give halo form
reaction.
(3) The overall
order of the reaction is usually 3.
(4) The Cannizzaro
reaction takes place very slowly when electron-donating groups are present. But the
reaction occurs at faster rates when electron withdrawing groups are present.
(5) Transfer of
hydride is rate determining step.
(6) In cannizaro reaction
kinetic isotopic effect is observed
Step-(1):
Step-(2):
(4) ILLUSTRATIONS &
EXAMPLES OF CANNIZZARO REACTION
(1) Formaldehyde is disproportionated
to formic acid and methyl alcohol in strong alkali.
(3) Furfural
gives furoic
acid and furfuryl alcohol in presence of strong alkali.
(2) Benzaldehyde
can be converted to benzoic acid and benzyl alcohol.
When
a mixture of two different aldehyde (alpha hydrogen less) like formaldehyde and a non enolizable
aldehyde (benzaldehyde) is treated with a strong base, the later is
preferentially reduced to alcohol while formaldehyde is oxidized to formic
acid. This variant is known as crossed Cannizzaro reaction.
Illustrative example: Benzyl alcohol
and formic acid are obtained when a mixture of benzaldehyde and formaldehyde is
treated with alkali.
Important
note:
(1) In cross cannizaro reaction if one of the reactant is formaldehyde, then
oxidation of formaldehyde take place , and reduction of another aldehyde take
place .The reason may be: the initial nucleophillic addition of hydroxide anion
is faster on formaldehyde as there are no electron donating groups on it.
(2) The preferential
oxidation of formaldehyde in crossed Cannizzaro reactions may be utilized in
the quantitative reduction of some aldehydes.
(6) INTRAMOLECULAR
CANNIZARO REACTION:
α-keto aldehydes can be converted to α-hydroxy
carboxylic acids by an intermolecular Cannizzaro reaction.
Illustrative Example: Phenylglyoxal
undergoes intramolecular
cannizzaro reaction by giving Mandelic acid
(α-hydroxyphenylacetic acid or 2-Hydroxy-2-phenylethanoic acid)