Search This Blog
Thursday, January 31, 2019
Structure of “Borazine/Borazole”/inorganic Benzene:
(1) Borazine is an inorganic compound with the chemical formula (B3N3H6).and also called Borazole. It is a heterocyclic compound, containing the 3-(BH) units and 3-(NH) units alternate.
(2) Borazine formed by reaction of B2H6 and NH3 in the ratio of 1:2 at room temperature.
(3) Borazine is isoelectronic and isostructural with benzene. For this reason borazine is known as “inorganic benzene”. Like benzene borazine has delocalized electrons and aromatic character. And it is a colourless liquid.
(4) In Borazine molecule nitrogen is more
electro negative than the boron. Nitrogen acquires partial negative charge and
boron acquires partial positive charge and back bonding take
place between boron and nitrogen.
(5) As compared with Benzene, Borazole/borazine is less stable and more reactive toward Electrophilic Aromatic Substitution reactions due to presence of polar B-N bond in it while in benzene all the C-C bonds are non polar.
(6) Borazine is a highly polar molecule due to high Electronegativity difference between Boron and Nitrogen.
Even though Borazine/Borazole and Benzene have same stricture their chemical properties are different.
(1) Organic benzene is C6H6 while
Inorganic benzene is Borazine having chemical formula B3N3H6
(2) The pi bonds in borazine are highly polarized than pi bonds in benzene due to high polarity (B-N polar bond) of Borazine molecules. Thus borazine is more nucleophillic (Negative) hence more reactive than benzene with respect to “Electrophic addition reactions”.
Here protonation(H+) take place at nitrogen atoms due to more electron density (more negative) and chlorine attack at boron atoms.
(3) Aromaticity of borazine is less than benzene because some delocalization in Borazine/Borazole is not complete as benzene. One reason is that nitrogen atom has more electronegativity than boron ,the electron density is higher at nitrogen atoms then boron. The electron density is determined by both sigma and pi bonds, both of which have polarity, but opposite directions, hence it is less reactive toward “Electrophilic substitution reactions” than Benzene.
(4) Borazine undergo polymerization when
strongly heated under vacuum and yield biborazonyl and naphthazine
whose structure are similar to biphenyl and naphthalene.
Related Questions:
Although anhydrous aluminium chloride is covalent but its aqueous solution is ionic in nature. Why?
Why Ga has small size than Al exceptionally
Why aqueous solution of borax reacts with two moles of acids ?
What is structure of solid Ortho Boric acid ?
What is the structure of trimetaboric acid and trimetaborate ion?
Why Borazine is more reactive than benzene towards Electrophic Aromatic substitution reactions ?
Why Borazine (B3N3H6) is also known as inorganic benzene ?.
Why B-F bond length in BF3 is shorter (130 pm) than B-F bond Iength in BF4- (143 pm)?. Explain.
Why B-F do not exist as dimer?. Explain.
Although anhydrous aluminium chloride is covalent but its aqueous solution is ionic in nature. Why?
Why Boric acid become strong acid in the presence of cis 1,2-diol or 1,3-diol ?
Four-center two-electron bond (4C-2e Bond): Structure of AlCl3:
What is the molecular formula of Borax ?
What is the difference between the structure of AlCl3 and diborane?
STRUCTURE OF DIBORANE :
Wednesday, January 30, 2019
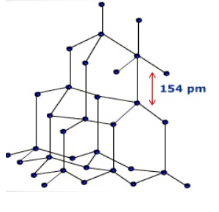
STRUCTURE OF DIAMOND :
STRUCTURE OF GRAPHITE:
STRUCTURE OF FULLERENCES :
Tuesday, January 29, 2019
REDOX TITRATIONS:
ILLUSTRATIVE EXAMPLE (7): Calculate the moles of KMnO4 required for Oxidation of 1.25 moles Cu2S.
ILLUSTRATIVE EXAMPLE (8): Calculate the Molarity of H2O2 if 11.2 ml H2O2 require 30 ml of 0.5 M K2Cr2O7 for its Oxidation . also calculate the volume of strength of H2O2.
ILLUSTRATIVE EXAMPLE (9): 696 gm of Fe2O3 and FeO reacts completely with 158 gm of KMnO4 in acidic medium . Calculate the composition of mixture.
ILLUSTRATIVE EXAMPLE (10): 829 gm of K2Cr2O7 and H2C2O4 reacts completely with 7/3 moles of K2Cr2O7 .
(1) Calculate the moles of each in mixture.
(2) Calculate the moles of NaOH required to react with above mixture.
ILLUSTRATIVE EXAMPLE (11): 50ml of KMnO4 is mixed with excess of KI the I2 liberated require 30 ml of 0.1M Na2S2O3 solution calculate the Molarity of KMnO4 solution.
ILLUSTRATIVE EXAMPLE (12): 50 Cm3 of 0.04 M K2Cr2O7 in acidic medium oxidized a sample of H2S gas to Sulphur . The volume of 0.03 M KMnO4 required to Oxidize the same amount of H2S gas to Sulphur, in acidic medium is.
ILLUSTRATIVE EXAMPLE (13): What volume of 0.4 M Na2S2O3 would be required to react with the I2 liberated by adding excess of KI to 50 ml of 0.2 M CuSO4? (Ans= 25 ml).