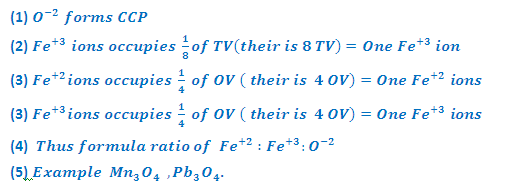Examples of sigma (σ) – donors:
Search This Blog
Sunday, November 29, 2020
What are sigma-donors ligands give the examples?
What are pi-acceptors ligands give the examples?
Examples of pi-acceptors ligands given as;
Related Questions;
(1) What type of ligands are known ?
(3) What is the common formula of “Prussian blue is” and “Turn bull's blue”?
(4) What is denticity of NO and NO+ ligands ?
(5) Why all the tetrahedral Complexes are high spin Complexes?
(6) Why Fe(CO)5 is colourless while Fe(bipy)(CO)3 is intensely purple in colour ?
(7) Why [Mn(H2O)6]+2 is colourless although in which Mn+2 ion had five unpaired electrons ?
(8) Why [FeF6]3– is colourless whereas [CoF6]3– is coloured?
(9) Why [Ni(CN)4]-2 is colourless while [Ni(H2O)4]-2 is colour although both have +2 oxidation state and 3d8 configuration ?
What are pi-donors ligands give the examples?
Ligands such as OR−, F−, and Cl− are π donors as a result of the lone pairs that are left after one lone pair has formed the M−L σ bond. Instead of stabilizing the dπ electrons of a d6 ion as does a π acceptor, these electrons are now destabilized by what is effectively a repulsion between two filled orbitals.
Examples of pi-donor ligands given as;
Related Questions;
(1) What type of ligands are known ?
(2) What is the common formula of “Prussian blue is” and “Turn bull's
blue”?
(3) What is denticity of NO and NO+ ligands ?
(5)Which of the Complex of the following pairs has the highest value of CFSE?
(6) Colour of Complexes due to charge transfer:
(7) Why violet colour of [Ti(H2O)6]Cl3 disapear (colourless) on heating heating ?
(9) Why [FeF6]3– is colourless whereas [CoF6]3– is coloured ?
(10) Why Fe(CO)5 is
colourless while Fe(bipy)(CO)3 is intensely purple in colour ?
Why all the
tetrahedral Complexes are high spin Complexes ?
What type of ligands are known ?
(1) What are pi-donors ligands give the examples?
Saturday, November 28, 2020
What are the inverse spinel structures?
INVERSE SPINEL STRUCTURE (Fe3O4-Magnetite):
What are the normal spinel structures?
NORMAL SPINEL (AB2O4 )
STRUCTURE:
Example of Spinel is a MgAl2O4.( mineral) In it oxide ions (O-2) are arranged in ccp with Mg+2 ions occupying tetrahedral voids and Al+3 ions in a set of octahedral voids.
Many ferrites (such as ZnFe2O4) also possess spinel structure. These are very
important magnetic materials and are used in telephone and memory loops in
computers.
Friday, November 27, 2020
Law of Isomorphism
Friday, November 20, 2020
What are the chemical formula and chemical name of Red Vitriol?
The chemical formula of Red Vitriol is CoSO4.xH2O (x=7) and chemical name is Cobalt sulphate.
What are the chemical formula and chemical name of "Mohar's Salt" ?
When saturated solutions of ferrous sulphate and al Ammonium sulphate are mixed and the solution is evaporated, we get the well known compound, Mohr’s salt.
What are the chemical formula and chemical name of Glauber salt ?
The chemical formula of Glauber salt is (Na2SO4·10H2O) and chemical name is hydrated sodium sulphate. it a colorless crystalline salt used in dyeing, as a cathartic, and in solar energy systems.
What are the chemical formula and chemical name of Epsom ?
The chemical formula of Epsom is MgSO4.xH2O (x=7) and chemical name is Magnesium sulphate.
What are the chemical formula and chemical name of Plaster of paris ?
The chemical formula of Plaster of paris is CaSO4.xH2O (x=1/2) and chemical name is Calcium sulphate hemihydrate.