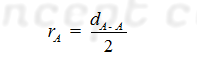(1) Stretching frequency of CO is inversely proportional to negative oxidation state of central metal atom
Search This Blog
Tuesday, December 10, 2024
What is synergic bonding , Bonding in metal Carbonyl , Bond order of M-C and C-O in synergic Bond
Sunday, August 15, 2021
Atomic Radius:
(1) The radius of an atom may be taken as the distance between atomic nucleus and the outermost shell of electrons of the atom.
(2) According
to the Heisenberg’s uncertainty principle the position of a moving electron cannot
be accurately determined. So the distance between the nucleus and the outermost
electron is uncertain.
(3) Atomic
radius can be determined indirectly from inter nuclear distance between the two
atoms in a gaseous diatomic molecule. This internuclear distance between the
two atoms is called bond length.
(4) The
internuclear distance between the two atoms can be measured by X– ray
diffraction or spectroscopic studies and also nuclear magnetic resonance (NMR)
spectrum.
(5) Atomic
radius depends on the type of chemical bond between atoms in a molecule. These
are:
(i) Covalent radius
(ii) Metallic radius or crystal radius
(iii) Vander Waal’s radius
(iv) Ionic radius or Collision radius
(6) Periodicity in
Atomic Radius and Ionic Radius:
Related Questions:
(1) What are the Amphoteric metals ? gives Examples.
(2) Name of total metalloids present in periodic table ?
(3) Total numbers of elements which are liquid at normal temperature is ?
(4) What is Mendeleev's periodic table ? give important features and draw back of Mendeleev's table.
(5) What is atomic density ? give the periodicity of atomic density in periods and groups.
(6) What is atomic volume ? and what is periodicity of atomic volume in groups and periods ?
(7) Why there are 2, 8 and 8 elements in first, second and third periodic of periods table respectively ? Explain.
(8) In alkali metal group which is the strongest reducing agent in aqueous solution and why?
(9) The electron affinity of sulphur is greater than oxygen. Why?
(10) The first ionization energy of carbon atom is greater than that of boron atom, whereas reverse is true for the second ionization energy. Explain.
(11) The electronegativities of B, Al, Ga are 2.0, 1.5, 1.6 respectively. The trend is not regular. Explain.
(12) Li2CO3 decomposes on heating but other alkali metal carbonates don’t. Explain.
(13) Of all noble metals, gold has got a relatively high electron affinity. Explain.
(14) What are the increasing order of ioni radii of first group elements in water ?
(15) What are the increasing order of molar conductivity of first group elements in water ?
Covalent radius:
Covalent radius: It is defined as one half of the distance between the nuclei (inter nuclear distance) of two covalently bonded like atoms in a homo nuclear diatomic molecule is called the covalent radius of that.
(A) For Homo atomic Molecules: The
covalent radius (rA) of atom A in a molecule A2 may be
given as:
The distance between nuclei of two single covalently bonded atoms in homo diatomic molecules is equal to the sum of covalent radii of both the atoms.
Illustrative
example (1): A given compound A2 whose total dA-A is 1.4
A0. The atomic (covalent) radius of an atom is.
Solution: We known that
(B)
For Heteroatomic Molecules: In a hetero diatomic molecules AB where the electro negativity of
atoms A and B are different, the experimental values of inter nuclear distance
dA-B is less than the theoretical value (rA +rB).
(1) Stevenson
& Schoemaker Equation (1941):
Covalent
radius of heterogeneous molecule like A-B etc determine by Stevenson &
Schromaker Equation, if atoms are formed different type of covalent bond i.e.
on atom is more electronegative than the other combined atom. Then the covalent
radius is calculated by the relation given by Stevenson & Schoemaker, given
as:
For a
diatomic Hetero molecule:
Bond
Length (lA-B) = rA + rB- 0.09(XA-XB)
Where XA=
Electronegativity of more electronegative atom
Where XB= Electronegativity of less electronegative atom
Illustrative examples
(1) The electronegativity of F and H are 4.0 and 2.1 respectively. The percentage ionic character in H and F bond is.
(3) A given compound AB whose electronegative difference is 1.9 . Atomic radius of A and B are 4 and 2 Angstroms the distance between A and B means dA-B is ?
(5) The C–C single bond length is 1.54 Angstroms and that of Cl–Cl is 1.98 Angstroms. If the electronegativity of Cl and C are 3.0 and 2.5 respectively, the C–Cl bond-length will be equal to ?
(2) Puling equation: If the electro negativities of the two atoms A and B are XA and XB respectively then
Bond Length (dA-B) =( rA +rB)-(C1XA-C2XB)
Where C1 and C2 are the Stevenson’s coefficients for atoms A and B respectively
Related Questions:
(1) What are the Amphoteric metals ? gives Examples.
(2) Name of total metalloids present in periodic table ?
(3) Total numbers of elements which are liquid at normal temperature is ?
(4) What is Mendeleev's periodic table ? give important features and draw back of Mendeleev's table.
(5) What is atomic density ? give the periodicity of atomic density in periods and groups.
(6) What is atomic volume ? and what is periodicity of atomic volume in groups and periods ?
(7) Why there are 2, 8 and 8 elements in first, second and third periodic of periods table respectively ? Explain.
(8) In alkali metal group which is the strongest reducing agent in aqueous solution and why?
(9) The electron affinity of sulphur is greater than oxygen. Why?
(10) The first ionization energy of carbon atom is greater than that of boron atom, whereas reverse is true for the second ionization energy. Explain.
(11) The electronegativities of B, Al, Ga are 2.0, 1.5, 1.6 respectively. The trend is not regular. Explain.
(12) Li2CO3 decomposes on heating but other alkali metal carbonates don’t. Explain.
(13) Of all noble metals, gold has got a relatively high electron affinity. Explain.
(14) What are the increasing order of ioni radii of first group elements in water ?
(15) What are the increasing order of molar conductivity of first group elements in water ?
Friday, August 6, 2021
Why Back bonding does not take place in P(SiH3)3 instead of phosphorus has lone pair and Silicon has vacant d-orbital but in N(SiH3)3 molecule back bonding does take place?
Conditions for back bonding:
(1) Both of the atoms bonded with back Bonding are must be present in 2nd-2nd or 2nd-3rd period. 4rth period onward back bonding does not take place.
(2) One of
the atoms has lone pair (donor atom) and another (acceptor atom) have vacant
Orbital and direction of back Bonding depends upon vacant Orbital.
(3) The donor atom must have localized donatable electron pair and there should be inter electronic repulsion (smaller size). In general these are later half second period P - block elements
(F, O, N and C).
(4) The acceptor atom must have low energy empty orbital which
generally are np or nd orbitals. Small and similar size orbital’s favour overlap.
(5)
Back bonding is a weak pi bond thus only effective overlapping will be form
back bonding.
(6) Back bonding is found to be effective and considerable in following type of overlapping.
(i)
2p-2p
(ii) 2p-3p
(iii) 2p-3d
(7) The extent of overlapping order is [2p-2p> 2p-3d >2p-3p]
(8) dx2-y2 and dx2 Orbital’s does not participate in back Bonding.
Solution:
In case of trisilyl phosphine [P(SiH3)3] Phosphorous (P) being larger in atomic
size so it does not face much interelectronic repulsion and so, not much eager
to donate it’s loan pair electron and comfortable with it. So, it will not
donate to Si atom in this case. Thus, back bonding will not take place in trisilyl
phosphine [P(SiCH3)3].
But in the case of trisilyl amine [N(SiH3)3],
the nitrogen (N)
atom is
smaller in atomic size leading to high interelectronic repulsion, so it
wants ease by donating it’s loan pair electron to other atoms near to
it. And Si has vacant d-orbital, so needy for it. Since
both conditions are follow here, hence back
bonding will take place.
(1) Why trimethylamine amine ( N(CH3)3) is tetrahedral while trisilyl amine (N(SiH3)3) planner.?
(2) Why trimethyl amine {(CH3)3 N:} is pyramidal while trisilyl amine {(SiH3)3N:} is trigonal planer?
(6) Arrange the silicon halides into decreasing order of Lewis acids Character? SiF4, SiCl4, SiBr4, SiI4
Thursday, August 5, 2021
Metallic radius or Crystal radius:
Metallic radius or Crystal radius:
(1) The term crystal radius is used to denote the
size of atoms in metal.
(2) Metal atoms are closely packed spheres in
metallic crystal. The metal atoms are supposed to touch one another in crystal.
(3) Metallic radius is defined as one half the distances
between the centres of the nuclei of two atoms in a metallic crystal.
(4) Metallic radius is determined by X-ray diffraction
method.
(5) Metallic radii are about 10 to 15 % higher
than the single bond covalent raddi of those elements. Thus single bond covalent radius is smaller than the metallic
radius due to the no overlapping of atomic orbital in metallic bond.
Vander Waal’s radius > Metallic radius > Covalent radius
(6) For the
simplicity the term atomic radius is used for covalent radius as well as
metallic radius depending on whether the element is a non-metal or metal. However,
the atomic radii of inert gases are expressed in the terms of Vander Waal’s
radii.
(7) Metallic
radius is inversely proportional to the metallic bond strength.
(8) More
metallic radius –loose crystal packing-less bond strength. (BCC)
(9) Less
metallic radius –Tight crystal packing (FCC) - high bond strength.(HCP)
(10) For
non-metal, atomic radius means covalent radius.
(11) For metal,
atomic radius means metallic radius.
(12) For inert gases, atomic radius means Vander Waal’s radius.
Vander Waal’s radius or Collision radius:
(1) What are the Amphoteric metals ? gives Examples.
(2) Name of total metalloids present in periodic table ?
(3) Total numbers of elements which are liquid at normal temperature is ?
(4) What is Mendeleev's periodic table ? give important features and draw back of Mendeleev's table.
(5) What is atomic volume ? and what is periodicity of atomic volume in groups and periods ?
(6) Why there are 2, 8 and 8 elements in first, second and third periodic of periods table respectively ? Explain.
(7) In alkali metal group which is the strongest reducing agent in aqueous solution and why?
(8) The electron affinity of sulphur is greater than oxygen. Why?
(9) The first ionization energy of carbon atom is greater than that of boron atom, whereas reverse is true for the second ionization energy. Explain.
(10) The electronegativities of B, Al, Ga are 2.0, 1.5, 1.6 respectively. The trend is not regular. Explain.
(11) Li2CO3 decomposes on heating but other alkali metal carbonates don’t. Explain.
(12) Of all noble metals, gold has got a relatively high electron affinity. Explain.
(13) Ionization energy of Boron is smaller than Beryllium even though effective nuclear charge is higher?
(14) What are the increasing order of ioni radii of first group elements in water ?
(15) What are the increasing order of molar conductivity of first group elements in water ?
Friday, July 23, 2021
What is the shielding constant (S) experienced by a 3d electron in the bromine atom?
Step (1): Write the electronic configuration of Bromine in the appropriate form.
Br: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5
Br: (1s2)(2s2,2p6)(3s2,3p6)(3d10)(4s2,4p5)
Step (2): Use the Slater Rule to calculate the shielding constant for the electron. And ignore the group of electron to the right of the 3d electrons, these electrons do not contribute to the shielding constant.
(S)3d
= (d electrons-1)x 0.35+ (remaining electrons)x1.00= 21.15
(S)3d
= (10-1)x 0.35+ (18)x1.00= 21.15
Solved Questions: