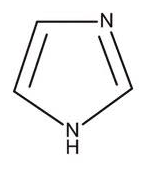
Imidazole
and its derivatives form an interesting and important class of heterocyclic aromatic
amines.
The basic
strength of imidazole is approximately 100 times more basic than pyridine.
Because protonation of imidazole yields an ion that is stabilized by the
electron delocalization represented in the resonance structures given as below:
As
seen in above figure the electrostatic potential map of the conjugate acid of
imidazole (imidazolium ion) is consistent with the resonance description that
shows both nitrogens as equivalent.
Related Questions: