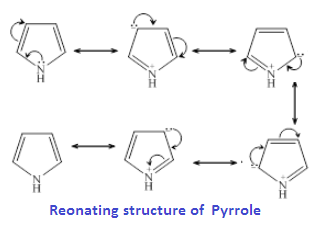
While in case pyridine
already has a stable conjugated system of three double bonds in an aromatic ring,
like benzene. Hence the lone pair electrons on the N atom in pyridine is
localized and more available for donation
and easily donated to a H+ ions. Therefore, pyridine is a stronger base than Pyrrole.
Related Questions: