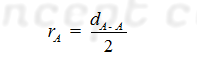Covalent radius: It is defined as one half of the distance between the nuclei (inter nuclear distance) of two covalently bonded like atoms in a homo nuclear diatomic molecule is called the covalent radius of that.
(A) For Homo atomic Molecules: The
covalent radius (rA) of atom A in a molecule A2 may be
given as:
The distance between nuclei of two single covalently bonded atoms in homo diatomic molecules is equal to the sum of covalent radii of both the atoms.
Illustrative
example (1): A given compound A2 whose total dA-A is 1.4
A0. The atomic (covalent) radius of an atom is.
Solution: We known that
(B)
For Heteroatomic Molecules: In a hetero diatomic molecules AB where the electro negativity of
atoms A and B are different, the experimental values of inter nuclear distance
dA-B is less than the theoretical value (rA +rB).
(1) Stevenson
& Schoemaker Equation (1941):
Covalent
radius of heterogeneous molecule like A-B etc determine by Stevenson &
Schromaker Equation, if atoms are formed different type of covalent bond i.e.
on atom is more electronegative than the other combined atom. Then the covalent
radius is calculated by the relation given by Stevenson & Schoemaker, given
as:
For a
diatomic Hetero molecule:
Bond
Length (lA-B) = rA + rB- 0.09(XA-XB)
Where XA=
Electronegativity of more electronegative atom
Where XB= Electronegativity of less electronegative atom
Illustrative examples
(1) The electronegativity of F and H are 4.0 and 2.1 respectively. The percentage ionic character in H and F bond is.
(3) A given compound AB whose electronegative difference is 1.9 . Atomic radius of A and B are 4 and 2 Angstroms the distance between A and B means dA-B is ?
(5) The C–C single bond length is 1.54 Angstroms and that of Cl–Cl is 1.98 Angstroms. If the electronegativity of Cl and C are 3.0 and 2.5 respectively, the C–Cl bond-length will be equal to ?
(2) Puling equation: If the electro negativities of the two atoms A and B are XA and XB respectively then
Bond Length (dA-B) =( rA +rB)-(C1XA-C2XB)
Where C1 and C2 are the Stevenson’s coefficients for atoms A and B respectively
Related Questions: