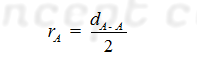Water is a polar compound with a high dielectric constant (81).
Hence, it decreases the electrostatic forces of attraction acting between the
oppositely charged ions in an electrovalent compound. Therefore, the ions are
separated and dissolution occurs. Dissolution of electrolytes in water is also
favoured due to hydration of ions.
Search This Blog
Sunday, September 26, 2021
Water is generally a good solvent for ionic compounds. Why?
Sunday, August 15, 2021
Oxy acids of Sulphur:
Oxidation state of sulphur atom and respective oxy-acids:
Oxidation state | Series name | Prefix of acid | Name of acids |
(+2) | “us”series | Hypo | Hyposulphurous acid |
(+4) | “us”series | ……. | Sulphurous acid |
(+5) | “ic”series | Hypo | Hyposulphuric acid |
(+6) | “ic”series | ……. | Sulphuric acid |
(+7)/(+8)Cal/ava | “ic”series | Peroxy | Peroxysulphuric acid |
Important Note:
(1) Ortho and meta form of sulphurous and sulphuric acid not exist but both have pyro form only.
(2) Hyposulphurous acid start thionous series and while hyposulphuric acid start thionic series;
(1) Sulphurous Series:
(A) Sulphurous acid:
(B) Hyposulphurous acid(H2SO2/S(OH)2):
(C) Thiosulphurous acid(H2S2O2):
(D) Pyrosulphurous acid(H2S2O5=H2SO3+SO2):
(2) Sulphuric acid series:
(A) Sulphuric acid(H2SO4):
(B) Pyrosulphuric acid(H2S2O7=H2SO4+SO3): Oleum:
(C) Peroxomonosulphuric acid(H2SO5):Caro’s acid:
(D) Peroxodisulphuric acid(H2S2O8): Marshall’s acid:
(3) Thionous acid series
(4) Thionic acid series:
(A) Dithionic acid(H2S2O6):
(B) Trithionic acid (H2S3O6):
(C) Tetrathionic acid(H2S4O6):
Related Topics:
(2) What are "Ortho" or "Meta" oxyacids?
(3) What are "pyro" oxy acids?
(6) Prefix “Hypo-acids”, and “Per-acids”:
Related Questions:
(1) What is Use of Boric Acid?
(2) What is use of Orthoboric acids?
(3) What is basicity of "Boric acid" ?
(4) Why Boric acid exist in solid state ?
(5) What is structure of solid Ortho Boric acid ?
(6) What is effect of heat on Borax?
(7) What is the structure of trimetaboric acid and trimetaborate ion?
(8) What is the Sodium per borate ,give the structure and its uses?
(9) Why aqueous solution of borax reacts with two moles of acids ?
(10) What is the molecular formula of Borax ?
(11) Why Boric acid become strong acid in the presence of cis 1,2-diol or 1,3-diol ?
(12) Why Borazine is more reactive than benzene towards Electrophic Aromatic substitution reactions ?
(13) Why Borazine (B3N3H6) is also known as inorganic benzene ?.
Why radii of Ar is greater than the radii of chlorine?
In chlorine, the radii mean the atomic or covalent radii which is actually half the intermolecular distance between 2 atoms whereas in Argon the radii means the Vander waals radii as Argon is not a diatomic molecule. Vander Waal’s radii are actually half the distance between adjacent molecule. So Vander Waal’s radii being larger than atomic radii, Argon has a larger radii than chlorine.
(1) What are the Amphoteric metals ? gives Examples.
(2) Name of total metalloids present in periodic table ?
(3) Total numbers of elements which are liquid at normal temperature is ?
(4) What is Mendeleev's periodic table ? give important features and draw back of Mendeleev's table.
(5) What is atomic volume ? and what is periodicity of atomic volume in groups and periods ?
(6) Why there are 2, 8 and 8 elements in first, second and third periodic of periods table respectively ? Explain.
(7) In alkali metal group which is the strongest reducing agent in aqueous solution and why?
(8) The electron affinity of sulphur is greater than oxygen. Why?
(9) The first ionization energy of carbon atom is greater than that of boron atom, whereas reverse is true for the second ionization energy. Explain.
(10) The electronegativities of B, Al, Ga are 2.0, 1.5, 1.6 respectively. The trend is not regular. Explain.
(11) Li2CO3 decomposes on heating but other alkali metal carbonates don’t. Explain.
(12) Of all noble metals, gold has got a relatively high electron affinity. Explain.
(13) Ionization energy of Boron is smaller than Beryllium even though effective nuclear charge is higher?
(14) What are the increasing order of ioni radii of first group elements in water ?
(15) What are the increasing order of molar conductivity of first group elements in water ?
Atomic Radius:
(1) The radius of an atom may be taken as the distance between atomic nucleus and the outermost shell of electrons of the atom.
(2) According
to the Heisenberg’s uncertainty principle the position of a moving electron cannot
be accurately determined. So the distance between the nucleus and the outermost
electron is uncertain.
(3) Atomic
radius can be determined indirectly from inter nuclear distance between the two
atoms in a gaseous diatomic molecule. This internuclear distance between the
two atoms is called bond length.
(4) The
internuclear distance between the two atoms can be measured by X– ray
diffraction or spectroscopic studies and also nuclear magnetic resonance (NMR)
spectrum.
(5) Atomic
radius depends on the type of chemical bond between atoms in a molecule. These
are:
(i) Covalent radius
(ii) Metallic radius or crystal radius
(iii) Vander Waal’s radius
(iv) Ionic radius or Collision radius
(6) Periodicity in
Atomic Radius and Ionic Radius:
Related Questions:
(1) What are the Amphoteric metals ? gives Examples.
(2) Name of total metalloids present in periodic table ?
(3) Total numbers of elements which are liquid at normal temperature is ?
(4) What is Mendeleev's periodic table ? give important features and draw back of Mendeleev's table.
(5) What is atomic density ? give the periodicity of atomic density in periods and groups.
(6) What is atomic volume ? and what is periodicity of atomic volume in groups and periods ?
(7) Why there are 2, 8 and 8 elements in first, second and third periodic of periods table respectively ? Explain.
(8) In alkali metal group which is the strongest reducing agent in aqueous solution and why?
(9) The electron affinity of sulphur is greater than oxygen. Why?
(10) The first ionization energy of carbon atom is greater than that of boron atom, whereas reverse is true for the second ionization energy. Explain.
(11) The electronegativities of B, Al, Ga are 2.0, 1.5, 1.6 respectively. The trend is not regular. Explain.
(12) Li2CO3 decomposes on heating but other alkali metal carbonates don’t. Explain.
(13) Of all noble metals, gold has got a relatively high electron affinity. Explain.
(14) What are the increasing order of ioni radii of first group elements in water ?
(15) What are the increasing order of molar conductivity of first group elements in water ?
Covalent radius:
Covalent radius: It is defined as one half of the distance between the nuclei (inter nuclear distance) of two covalently bonded like atoms in a homo nuclear diatomic molecule is called the covalent radius of that.
(A) For Homo atomic Molecules: The
covalent radius (rA) of atom A in a molecule A2 may be
given as:
The distance between nuclei of two single covalently bonded atoms in homo diatomic molecules is equal to the sum of covalent radii of both the atoms.
Illustrative
example (1): A given compound A2 whose total dA-A is 1.4
A0. The atomic (covalent) radius of an atom is.
Solution: We known that
(B)
For Heteroatomic Molecules: In a hetero diatomic molecules AB where the electro negativity of
atoms A and B are different, the experimental values of inter nuclear distance
dA-B is less than the theoretical value (rA +rB).
(1) Stevenson
& Schoemaker Equation (1941):
Covalent
radius of heterogeneous molecule like A-B etc determine by Stevenson &
Schromaker Equation, if atoms are formed different type of covalent bond i.e.
on atom is more electronegative than the other combined atom. Then the covalent
radius is calculated by the relation given by Stevenson & Schoemaker, given
as:
For a
diatomic Hetero molecule:
Bond
Length (lA-B) = rA + rB- 0.09(XA-XB)
Where XA=
Electronegativity of more electronegative atom
Where XB= Electronegativity of less electronegative atom
Illustrative examples
(1) The electronegativity of F and H are 4.0 and 2.1 respectively. The percentage ionic character in H and F bond is.
(3) A given compound AB whose electronegative difference is 1.9 . Atomic radius of A and B are 4 and 2 Angstroms the distance between A and B means dA-B is ?
(5) The C–C single bond length is 1.54 Angstroms and that of Cl–Cl is 1.98 Angstroms. If the electronegativity of Cl and C are 3.0 and 2.5 respectively, the C–Cl bond-length will be equal to ?
(2) Puling equation: If the electro negativities of the two atoms A and B are XA and XB respectively then
Bond Length (dA-B) =( rA +rB)-(C1XA-C2XB)
Where C1 and C2 are the Stevenson’s coefficients for atoms A and B respectively
Related Questions: