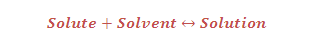Search This Blog
Wednesday, April 29, 2020
0.0833 moles of a carbohydrate having empirical formula of CH2O contains one gram hydrogen, find molecular formula
Given EF= CH2O then mass=30
And carbohydrate(CH2O) contains 1gram hydrogen:
From EF it is obvious that 2 grams hydrogen present in 30 grams then 1grams hydrogen present in 15 grams corbohydrate.
Number of moles = Given mass/M.Mass
0.0833 =15/M.mass
Then M.mass=180
We Must needed
Molecular Formula(MF) = n× Empirical Formula (EF)
MF=6(CH2O) = C6H12O6
Where n = Molecular mass/Empirical mass
n= 180/30=6
Monday, April 27, 2020
Which carbocation is more stable : Benzyl or Tertiary?
Some time it is dilemma tertiary is more stable than Benzylic or benzylic is more stable than tertiary carbocation.
But their is two case (1) Primary benzylic carbonation is less stable than 3° carbocation because the tertiary carbocation is stablized due to +I effect and +H effect, while benzylic carbocation stabilised by +R effect only thus tertiary become more stable than primary Benzylic .
In (2) case of secondary and tertiary benzylic carbocation become more stable than tertiary because now +H effect also operates along with +R effect.
Similar Questions:
Saturday, April 25, 2020
What is the Raoult's law and it's application ?
RAOULT'S LAW:
(1) Vapour pressure of a number of binary solutions of volatile liquids such as benzene and toluene at constant temperature gave the following generalization which is known as the Raoult's law.
(2) Raoult's law states that "The partial pressure of any volatile component of a solution at any temperature is equal to the vapour pressure of the pure component multiplied by the mole fraction of that component in the solution
(A) Vapour pressure of liquid-liquid Solution:
(3) Suppose a binary solution contains nA moles of a volatile liquid A and nB moles of a volatile liquid B, if PAand PB are partial pressure of the two liquid components, the according to Raoult's law
(4) If the vapour behaves like an ideal gas, thenaccording to Dalton's law of partial pressures, the total pressure P is given by
Graphical representation of Raoult's law:
(5) The relationship between vapour pressure and mole fraction of an ideal solution at constant temperature is shown. The dashed lines 1 and 2 represent the partial pressure of the components. The total vapour pressure is given by 3rd line in the above figure.
(B) Vapour pressure of Solid-liquid Solution:
(1) Vapor pressure, when a small amount of a non-volatile solute (solid) is added to the liquid (solvent). It is found that the vapour pressure of the solution is less than that of the pure solvent.
(2) The lowering of vapour pressure is due to the fact that the solute particles occupy a certain surface area and evaporation takes place from the surface only. and
(3) The particles of the solvent will have a less tendency to change into vapour i.e. the vapour pressure of the solution will be less than that of the pure solvent and it is termed as lowering of vapour pressure.
For a solution of non-volatile solute with volatile solvent.
What is Vapour pressure of liquid ?
VAPOUR PRESSURE:
(1) If a sample of water in its liquid phase is placed in an empty container, some of it will vaporize to form gaseous of water. This change is called evaporation.
(2) The pressure exerted by the vapour (molecules in the vapour phase) over the surface of the liquid at the equilibrium at given temperature is called the vapour pressure of the liquid.
OR
(3) It is the pressure exerted by the vapour when vapours are equilibrium with the liquid.
(4) The pressure exerted by vapours is called unsaturated vapour pressure or partial vapour at non equilibrium condition.
Factors affecting vapour pressure:
(A) Temperature:.
(1) The temperature at which the vapour pressure of the liquid becomes equal to the atmospheric pressure is called its boiling point.
(2) Vapour pressure is directly proportional to theTemperature so that on increasing temperature the rate of evaporation increases and rate of condensation decreases and hence vapour pressure increases.
(3) The dependence of vapour pressure and temperature is given by CLASIUS CLAPERON equation.
(4) Vapour pressure of a particular liquid system is only the function of temperature only. It is independent from all other factors like surface area, amount of liquid, available space etc.
(A) Nature of liquid:
Vapour pressure of liquid =1/the strength of intermolecular forces acting between molecules
For example: CCl4 has higher vapour pressure because of the weak intermolecular forces acting between its molecules than water which has stronger intermolecular forces acting between water molecules of volatile liquid has lower boiling point than a non-volatile liquid.
Note:
(1) Relative lowering of vapour pressure of a solvent is a colligative property equal to the vapour pressure of the pure solvent minus the vapour pressure of the solution.
(2) For example: water at 20°C has a vapour pressure of 17.54 mmHg. Ethylene glycol is a liquid whose vapour pressure at 20°C is relatively low, an aqueous solution containing 0.010 mole fraction of ethylene glycol has a vapour pressure of 17.36 mmHg. Thus the vapour pressure lowering, DP = 17.54 mmHg ¾ 17.36 mmHg = 0.18 mmHg.
Explain factors affecting solubility of a substance .
Factors affecting Solubility:
Earlier we have observed that solubility of one substance into another depends on the nature of the substances. In addition to these variables, two other parameters, i.e., temperature and pressure also control this phenomenon.
(1) Effect of temperature:
The solubility of a solid in a liquid is significantly affected by temperature changes. Consider the equilibrium exist between dissolution and crystallisation. This, being dynamic equilibrium, must follow Le Chateliers Principle. In general…….
Earlier we have observed that solubility of one substance into another depends on the nature of the substances. In addition to these variables, two other parameters, i.e., temperature and pressure also control this phenomenon.
(1) Effect of temperature:
The solubility of a solid in a liquid is significantly affected by temperature changes. Consider the equilibrium exist between dissolution and crystallisation. This, being dynamic equilibrium, must follow Le Chateliers Principle. In general…….
(i) If in a nearly saturated solution, the dissolution process is endothermic (Δsol H > 0), the solubility should increase with rise in temperature and
(ii) If it is exothermic (Δsol H > 0) the solubility should decrease. These trends are also observed experimentally.
(2) Effect of Pressure:
Pressure does not have any significant effect on solubility of solids in liquids. It is so because solids and liquids are highly incompressible and practically remain unaffected by changes in pressure.
(2) Effect of Pressure:
Pressure does not have any significant effect on solubility of solids in liquids. It is so because solids and liquids are highly incompressible and practically remain unaffected by changes in pressure.
(2) Solubility of gas in Liquid:
Many gases dissolve in water. Oxygen dissolves only to a small extent in water. It is this dissolved oxygen which sustains all aquatic life. On the other hand, hydrogen chloride gas (HCl) is highly soluble in water. Solubility of gases in liquids is greatly affected by pressure and
temperature.
Many gases dissolve in water. Oxygen dissolves only to a small extent in water. It is this dissolved oxygen which sustains all aquatic life. On the other hand, hydrogen chloride gas (HCl) is highly soluble in water. Solubility of gases in liquids is greatly affected by pressure and
temperature.
Factors affecting Solubility:
(1) Effect of Pressure:
The solubility of gases increase with increase of pressure. For solution of gases in a solvent, consider a solution is act as system and that system to be in astate of dynamic equilibrium, i.e., under these conditions rate of gaseous particles entering and leaving the solution phase is the same. Now increase the pressure over the solution phase by compressing the gas to a smaller volume, this will increase the number of gaseous particles per unit volume over the solution and also the rate at which the gaseous particles are striking the surface of solution to enter it. The solubility of the gas will increase until a new equilibrium is reached resulting in an increase in the pressure of a gas above the solution and thus its solubility increases.
What is solubility ?
SOLUBILITY OF GASES AND HENRY'S LAW:
Solubility:
Solubility of a substance is its maximum amount that can be dissolved in a specified amount of solvent at a specific temperature. It depends upon the nature of solute and solvent as well as temperature and pressure.
Unit: Unit of Solubility is gm/litre or Mole/Litre
(A) Solubility of Solid in a Liquid:
Every solid does not dissolve in a given liquid. While sodium chloride and sugar dissolve readily in water, naphthalene and anthracene do not. On the other hand, naphthalene and anthracene dissolve readily in benzene but sodium chloride and sugar do not.
Every solid does not dissolve in a given liquid. While sodium chloride and sugar dissolve readily in water, naphthalene and anthracene do not. On the other hand, naphthalene and anthracene dissolve readily in benzene but sodium chloride and sugar do not.
(1) It is clear observed that polar solutes dissolve in polar solvents and non polar solutes in non-polar solvents.
(2) In general, a solute dissolves in a solvent if the intermolecular interactions are similar in the two or we may say like dissolves like.
(3) Dissolution: When a solid solute is added to the solvent, some solute dissolves and its concentration increases in solution. This process is known asdissolution.
(4) Crystallisation: Some solute particles in solution collide with the solid solute particles and get separated out of solution. This process is known as crystallisation.
"A stage is reached when the two processes (dissolution and crystallisation) occur at the same rate. Under such conditions, number of solute particles going into solution will be equal to the solute particles separating out and a state of dynamic equilibrium is reached.
At this stage the concentration of solute in solution will remain constant under the given conditions, i.e., temperature and pressure. Similar process is followed when gases are dissolved in liquid solvents.
(5) Saturated solution: Such a solution in which no more solute can be dissolved at the same temperature and pressure is called a saturated solution.
(6) Unsaturated solution: The Solution in which more solute can be dissolved at the same temperature.
(7) The solution which is in dynamic equilibrium with undissolved solute is the saturated solution and contains the maximum amount of solute dissolved in a given amount of solvent. Thus, the concentration of solute in such a solution is its solubility.
What are the Application of Henry’s Law ? And explain with examples.
Henry's law finds several applications in industry and explains some biological phenomena Notable among these are:
(1) To increase the solubility of CO2 in soft drinks and soda water, the bottle is sealed under high pressure.
(2) To minimize the painful effects accompanying the decompression of deep sea divers, oxygen diluted with less soluble helium gas is used as breathing gas.
(3) In lungs, where oxygen is present in air with high partial pressure, haemoglobin combines with oxygen to form oxyhaemoglobin. In tissues where partial pressure of oxygen is low, oxyhaemoglobin releases oxygen for utilization in cellular activities.
(1) At High Pressure:
Scuba divers must cope with high concentrations of dissolved gases while breathing air at high pressure underwater. Increased pressure increases the solubility of atmospheric gases in blood. When the divers come towards surface, the pressure gradually decreases. This releases the dissolved gases and leads to the formation of bubbles of nitrogen in the blood. This blocks capillaries and creates a medical condition known as bends, which are painful and dangerous to life.
To avoid bends, as well as, the toxic effects of high concentrations of nitrogen in the blood, the tanks used by scuba divers are filled with air diluted with helium (11.7% helium, 56.2% nitrogen and 32.1% oxygen).
(2) At Low Pressure: At high altitudes the partial pressure of oxygen is less than that at the ground level. This leads to low concentrations of oxygen in the blood and tissues of people living at high altitudes or climbers. Low blood oxygen causes climbers to become weak and unable to think clearly, symptoms of a condition known as anoxia.
(2) At Low Pressure: At high altitudes the partial pressure of oxygen is less than that at the ground level. This leads to low concentrations of oxygen in the blood and tissues of people living at high altitudes or climbers. Low blood oxygen causes climbers to become weak and unable to think clearly, symptoms of a condition known as anoxia.
(2) Effect of temperature:
Solubility of gases in liquids decreases with rise in temperature. When dissolved, the gas molecules are present in liquid phase and the process of dissolution can be considered similar to condensation and heat is evolved in this process. We have known that dissolution process involves dynamic equilibrium and thus must follow Le Chatelier's Principle. As dissolution is an exothermic process, the solubility should decrease with increase of temperature.
Solubility of gases in liquids decreases with rise in temperature. When dissolved, the gas molecules are present in liquid phase and the process of dissolution can be considered similar to condensation and heat is evolved in this process. We have known that dissolution process involves dynamic equilibrium and thus must follow Le Chatelier's Principle. As dissolution is an exothermic process, the solubility should decrease with increase of temperature.
What is Henry's Law of Solubility ?
Henry's Law:
(1) The solubility of a gas in a liquid is determined by several factors. In addition to the nature of the gas and the liquid, solubility of the gas depends on the temperature and pressure of the system.
(2) The solubility of a gas in a liquid is governed by Henry's law which states that the solubility of a gas in a liquid is directly proportional to the pressure of the gas.
(3) Dalton, a contemporary of Henry, also concluded independently that the solubility of a gas in a liquid solution is a function of the partial pressure of the gas. If we use the mole fraction of the gas in the solution as a measure of its solubility, then: Mole fraction of the gas in a solution is proportional to the partial pressure of the gas.
Or, partial pressure of the gas in solution = KH ´ mole fraction of the gas in solution
Here KH is Henry's law constant
p = KH X (Solute)
If we draw a graph between partial pressure of the gas versus mole fraction of the gas in solution, then we should get a plot of the straight line passing through origin.
Experimental result for the solubility of HCl gas in Cyclohexane at 93 K the slope of line is the Henry's law constant
Different gases have different KH values at the same temperature. This suggests that KH is a function of the nature of the gas. Table gives KH values of some common gases at specified temperature
Values of Henry's law constant (KH) for some selected gases in water:
It is obvious from figure that the higher the value of KHat a given pressure, the lower is the solubility of the gas in the liquid. It can be seen from table that KH value for both N2 and O2 increases with increase in temperature indicating that solubility of gases decreases with increase of temperature. It is due to this reason that aquatic species are more comfortable in cold waters rather than warm waters.
Friday, April 24, 2020
A 1.0 g impure sample containing [Zn(NH3)4CI2] and some inert impurity was treated with 16 ml of 1.0 M NaOH solution, where all the complex was converted into [Na2Zn(OH)4]. Ammonia formed is first boiled off and then the excess of base required 4 ml of 1.0 M HCI solution for complete neutralisation. After the neutralisation the solution is reacted with excess of AgNO3 solution. the mass of AgCI produced (in mg) is x. The value of x10 is.
Give your Answer........
Calculate the millimoles of SeO3(2−) in solution on the basis of following data: 70 ml of 60/M solution of KBrO3 was added to SeO3(2−) solution. The bromine evolved was removed by boiling and excess of KBrO3 was back titrated with 12.5 mL of 25/Msolution of NaAsO2. The reactions are given below. I. SeO3(2−) +BrO3(−) + H+→SeO42−+Br2+H2O II. BrO3(−) +AsO2(−) + H2O→Br(−) +AsO4(3−)+ H+
A six co-ordinate complex of formula CrCl3.6H2O has green colour. One litre of O.1M solution of the complex when treated with excess of AgNO3 gave 28.7 g of white precipitate. The formula of the complex is .... (1) [Cr(H2O)6]CI3 (2) [Cr(H2O)5CI]CI2.H2O (3) [Cr(H2O)4CI2]Cl.2H2O (4) [Cr(H2O)3Cl3].3H2O
SOLUTION:
Given 28.7 g of white ppt obtained is 28.7/147.5= 0.2 mole Agcl precipitate Since 0.1M complexgives 0.2 mole AgCl means 2Cl− are ionisable or two Cl- ion must be present out of coordination sphere so, complex is [Cr(H2O)5Cl]Cl2.H2O
Thursday, April 23, 2020
If N2 gas is bubbled through water at 293 K, how many millimoles of N2 gas would dissolve in 1 litre of water. Assume that N2exerts a partial pressure of 0.987 bar. Given that Henry’s law constant for N2 at 293 K is 76.84 kbar.
The composition of vapour over a binary ideal solution is determined by the composition of the liquid. If XA and YA are the mole-fraction of A in the liquid and vapour, respectively find the value of XA for which YA-XA has a minimum. What is the value of the pressure at this composition?
The vapour pressure of ethanol and methanol are 44.5 mm and 88.7 mm Hg respectively. An ideal solution is formed at the same temperature by mixing 60 g of ethanol with 40g of methanol. Calculate total vapour pressure of the solution.
Monday, April 20, 2020
The ammonia prepared by treating ammonium sulphate with calcium hydroxide is completely used by NiCl2.6H2O to form a stable coordination compound. Assume that both the reactions are 100% complete. If 1584 g of ammonium sulphate and 952g of NiCl2.6H2O are used in the preparation, the combined weight (in grams) of gypsum and the nickel-ammonia coordination compound thus produced is___. (Atomic weights in g mol–1: H = 1, N = 14, O = 16, S = 32, Cl = 35.5, Ca = 40, Ni = 59) (JEE-ADVANCED 2018 PAPER-2)
Galena (an ore) is partially oxidized by passing air through it at high temperature. After some time, the passage of air is stopped, but the heating is continued in a closed furnance such that the contents undergo self-reduction. The weight (in kg) of Pb produced per kg of O2 consumed is______ . (Atomic weights in g mol–1 : O = 16, S = 32, Pb = 207). (JEE-Advanced 2018 Paper-2)
Sunday, April 19, 2020
Calculate how much H2SO4 will be obtained from 400 gm of Oleum sample having labelling 104.5%?
SOLUTION: 104.5 % labelled means 100 Oleum sample required 4.5 gm water to completely destroyed free SO3 present in 100 gm sample
100 gm Oleum sample require 4.5 gm water to destroyed all free SO3
Weight of SO3 in destroyed by 18 gm water is =18/18x80= 80 gm
Weight of H2SO4 = 400-80= 320 gm present in 400 gm Oleum sample
Weight of H2SO4 newly formed is =18/18x98= 98 gm
Total Weight H2SO4 =320+98=418 gm
25 gm of Oleum sample required 2 gm of water ,find out the % labelling of sample .
SOLUTION: : 25 gm oleum required 2 gm water
1 gm require …………. 2/25 gm water
100 gm require ……..2/25x100=8 gm
Hence % labelling is 108%
100 gm of 120% labelled Oleum is diluted with 15 gm of water. determined the new % labelling of Oleum ?.
SOLUTION: : Wt of SO3 in Original Oleum
The amount of free SO3 destroyed by 15 gm water is added
% labelling(X) = 105 %
The amount of free SO3 destroyed by 4.5 gm water=15/18 x 80=66.66 gm
Wt of left SO3 =88.88-66.66=22.22 gm
Given Wt of (Free) SO3= 22.22 % Find % labelling (X)
% labelling(X) = 105 %
Subscribe to:
Posts (Atom)