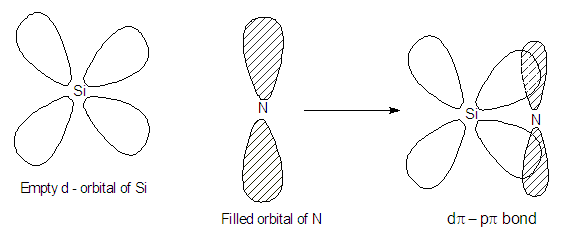
Catenation power of nitrogen is less because N-N bond is weaker than the single P-P bond. N-N single bond is weaker than P-P bond is due to smaller size of nitrogen atom there is stronger inter electronic repulsion between nitrogen atoms hence it form strong pπ–pπ multiple bond and become diatomic molecule.
Related Questions: