Search This Blog
Tuesday, December 31, 2019
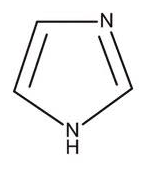
Imidazole is more basic than pyridine? Why?
Biological Important of Imidazole and structure:
Pyridine is almost 1 million times less basic than piperidine? Why?
Give an explanation for the fact that Guanidine NH=C(CH3)2 is a stronger base than most of amines?
Arrange in correct order of basic Character of aniline, pyrrol, pyridine and piperidine?
What is correct basicity order of pyridine, pyridazine, pyrimidine and pyrazine ?
Why pyridine is more basic than Pyrrole?
Why pyrimidine is less basic than pyridine?
Imidazole is more basic than pyridine? Why?
Biological Important of Imidazole and structure:
Pyridine is almost 1 million times less basic than piperidine? Why?
Cyclohexylamine amine is the stronger base than Aniline? Why?
Tetrahydroquinoline amine is the stronger base than Tetrahydroisoquinoline? Why?
Cyclohexylamine amine is the stronger base than Aniline? Why?
Tetrahydroquinoline amine is the stronger base than Tetrahydroisoquinoline? Why?
Are all the five bonds of PCl5 equivalent? Justify your answer.
Halides of Nitrogen Family:
Monday, December 30, 2019
Silianol (SiH3OH) is more acidic than methanol (CH3OH) why?
Related Questions:
(1) Although anhydrous aluminium chloride is covalent but its aqueous solution is ionic in nature. Why?
(2) Why Ga has small size than Al exceptionally
(3) Why aqueous solution of borax reacts with two moles of acids ?
(4) What is structure of solid Ortho Boric acid ?
(5) What is the structure of trimetaboric acid and trimetaborate ion?
(6) Why Borazine is more reactive than benzene towards Electrophic Aromatic substitution reactions ?
(7) Why Borazine (B3N3H6) is also known as inorganic benzene ?.
(8) Why B-F bond length in BF3 is shorter (130 pm) than B-F bond Iength in BF4- (143 pm)?. Explain.
(9) Why B-F do not exist as dimer?. Explain.
(10) Although anhydrous aluminium chloride is covalent but its aqueous solution is ionic in nature. Why?
(11) Why Boric acid become strong acid in the presence of cis 1,2-diol or 1,3-diol ?
Trisilyl amine, N(SiH3)3 is planar whereas trimethyl amines N(CH3)3 is pyramidal. Explain why?.
What is the molecular formula of Borax ?
Three-center four-electron Bridge bond (3C-4e Bond): Structure of AlCl3:
Related Questions:
(1) Why aqueous solution of AlCl3 is acidic in nature ?
(2) What happen when aq AlCl3 react with Acid or Base?
(3) Although anhydrous aluminium chloride is covalent but its aqueous solution is ionic in nature. Why?
(4) Why BF3 do not exist as dimer?. Explain.
(5) Why B-F bond length in BF3 is shorter (130 pm) than B-F bond Iength in BF4- (143 pm)?. Explain.
(6) B-F bond length in BF3 is shorter than B-F bond length in (BF4)- why?
(8) What is product of reaction between diborane (B2H6) and ammmonia (NH3)?
(9) Why methylation of Diborane (B2H6) replace four hydrogen only ?
(10) What is Use of Boric Acid?
(11) What is use of Orthoboric acids?
(12) What is basicity of "Boric acid" ?
(13) Why Boric acid exist in solid state ?
(14) What is structure of solid Ortho Boric acid ?
(15) What is effect of heat on Borax?
(16) What is the structure of trimetaboric acid and trimetaborate ion?
(17) What is the Sodium per borate ,give the structure and its uses?
(18) Why aqueous solution of borax reacts with two moles of acids ?
(19) What is the molecular formula of Borax ?
(20) Why Boric acid become strong acid in the presence of cis 1,2-diol or 1,3-diol ?
(21) Why Borazine is more reactive than benzene towards Electrophic Aromatic substitution reactions ?
(22) Why Borazine (B3N3H6) is also known as inorganic benzene ?.
(23) Four-center two-electron bond (4C-2e Bond): Structure of AlCl3:
(24) What is the difference between the structure of AlCl3 and diborane?
What is the difference between the structure of AlCl3 and diborane?
Related Questions:
(1) Why aqueous solution of AlCl3 is acidic in nature ?
(2) What happen when aq AlCl3 react with Acid or Base?
(3) Although anhydrous aluminium chloride is covalent but its aqueous solution is ionic in nature. Why?
(4) Why BF3 do not exist as dimer?. Explain.
(5) Why B-F bond length in BF3 is shorter (130 pm) than B-F bond Iength in BF4- (143 pm)?. Explain.
(6) B-F bond length in BF3 is shorter than B-F bond length in (BF4)- why?
(8) What is product of reaction between diborane (B2H6) and ammmonia (NH3)?
(9) Why methylation of Diborane (B2H6) replace four hydrogen only ?
(10) What is Use of Boric Acid?
(11) What is use of Orthoboric acids?
(12) What is basicity of "Boric acid" ?
(13) Why Boric acid exist in solid state ?
(14) What is structure of solid Ortho Boric acid ?
(15) What is effect of heat on Borax?
(16) What is the structure of trimetaboric acid and trimetaborate ion?
(17) What is the Sodium per borate ,give the structure and its uses?
(18) Why aqueous solution of borax reacts with two moles of acids ?
(19) What is the molecular formula of Borax ?
(20) Why Boric acid become strong acid in the presence of cis 1,2-diol or 1,3-diol ?
(21) Why Borazine is more reactive than benzene towards Electrophic Aromatic substitution reactions ?
(22) Why Borazine (B3N3H6) is also known as inorganic benzene ?.
(23) Four-center two-electron bond (4C-2e Bond): Structure of AlCl3:
(24) What is the difference between the structure of AlCl3 and diborane?
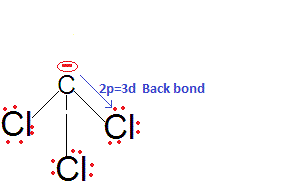
Chloroform is more acidic than fluoroform why?
We know that acidic strength of the acid also depends upon stability of conjugate bases, so for relative strength of acid, we need to check the relative stabilities of their conjugate bases.
CF3-, CCl3-, CBr3- CI3-
We are expecting the acidic strength haloform acids as CHF3, CHCl3, CHBr3, CHI3 in decreasing order. Because Fluorine is most electronegative atom so it would be stabilize CF3- more, as electronegativity decreases from F to I the stability of conjugate -ve ion would be but that is not correct the actual order is CHCl3 > CHF3 > CHBr3 > CHI3.
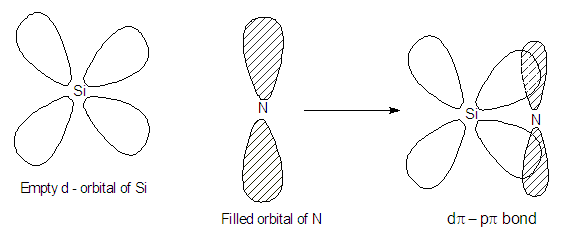
This is because there is effective back bonding in CCl3- and hence the negative charge partially gets stabilised by back donation to the vacant 3d orbitals of Cl. Thus, CHCl3 is a stronger acid than CHF3 and also among them due to 2pπ-3dπ back bonding.